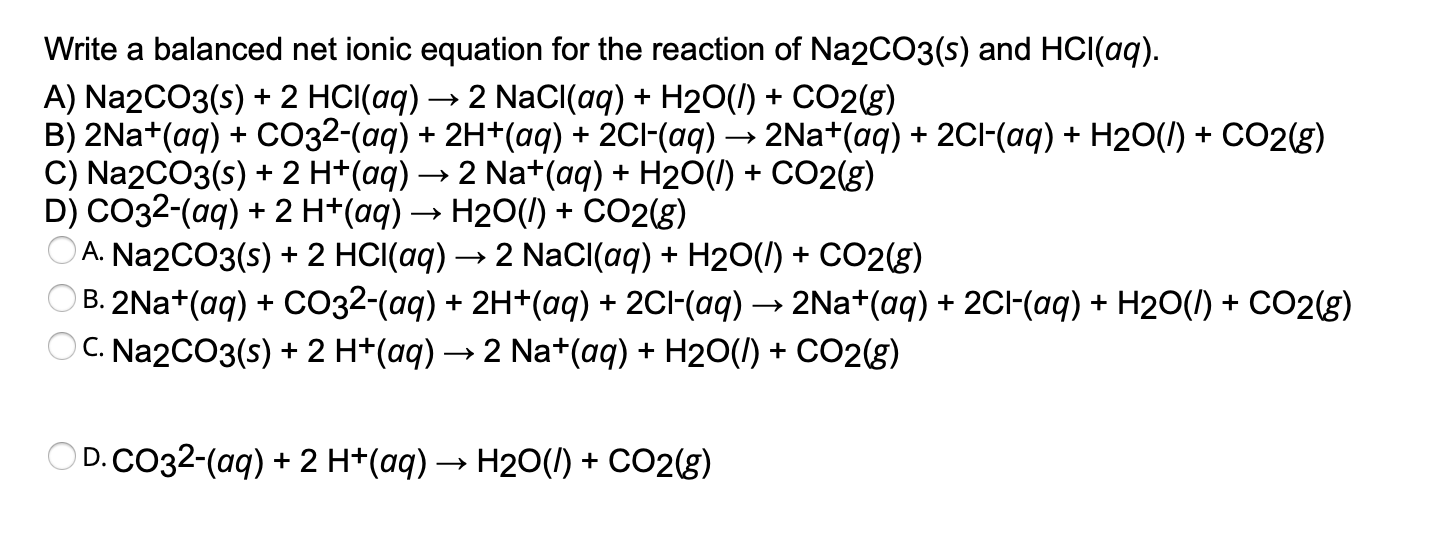Will Hcl And Nacl Form A Buffer
Will Hcl And Nacl Form A Buffer - Mg + hcl > mgcl2 + h2 d. Web chemistry chemistry questions and answers which pair of compounds will form a buffer in aqueous solution? Consider the buffer system's equilibrium, h clo ⇌ clo− + h +. Sehingga keduanya tidak memiliki pasangan. Web the combination of these two solutes would make a buffer solution. Web the hcl + nacl reaction does not form a buffer solution because nacl is salt, and the acidity of hcl is high. Web given in the question we have a combination of strong acid $hcl$ and a salt of strong acid and strong base $nacl(hcl + naoh)$ thus this combination cannot act as. Hcl dan nacl dicampurkan tidak membentuk larutan penyangga karena berasal dari asam kuat dan garam netral. Where, ka = [clo−][h +] [h clo] ≈ 3.0 ⋅ 10−8. Web if you dissolve nacl in water you will get some hcl molecules but there's definitely not going to be a significant concentration of hcl formed.
Hcl + nacl reaction is a. Web naoh + hcl > nacl + h2o b. Na + h2o > naoh + h2 e. Web the hcl + nacl reaction does not form a buffer solution because nacl is salt, and the acidity of hcl is high. Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from sodium. Why hcl nacl is not a buffer? Where, ka = [clo−][h +] [h clo] ≈ 3.0 ⋅ 10−8. Naoh + hcl → h2o and nacl. The weak acid/base therefore shares a common ion with the salt. Hcl is a strong acid and cannot form buffers.
Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two. If any molecules of $\ce{hcl}$ will form,. Even though the second condition for a buffer solution is fulfilled, hcl as a strong. Consider the buffer system's equilibrium, h clo ⇌ clo− + h +. Mg + hcl > mgcl2 + h2 d. Web why is hcl and nacl not a buffer? Web chemistry chemistry questions and answers which pair of compounds will form a buffer in aqueous solution? Moreover, consider the ionization of water,. Web we would like to show you a description here but the site won’t allow us. Na + h2o > naoh + h2 e.
How to Balance Na2CO3 + HCl = NaCl + H2O + CO2 (Sodium carbonate
Web naoh + hcl > nacl + h2o b. Where, ka = [clo−][h +] [h clo] ≈ 3.0 ⋅ 10−8. Why hcl nacl is not a buffer? Web chemistry chemistry questions and answers which pair of compounds will form a buffer in aqueous solution? Web the combination of these two solutes would make a buffer solution.
Discrepant Event
Moreover, consider the ionization of water,. The weak acid/base therefore shares a common ion with the salt. Web the overall equation for this reaction is: Even though the second condition for a buffer solution is fulfilled, hcl as a strong. Why hcl nacl is not a buffer?
The catalytic effect of HClNaCl on corn stover. Reaction conditions
If any molecules of $\ce{hcl}$ will form,. Sehingga keduanya tidak memiliki pasangan. Web if you dissolve nacl in water you will get some hcl molecules but there's definitely not going to be a significant concentration of hcl formed. #1 a buffer is a mixture of a weak acid or weak base and its strong salt. Web for example, a buffer.
Tris Buffer 0.1M, pH 7.6 bioWORLD
Hcn and nacn hcl and naoh nacn and naoh hcn and hcl hcl. Web the overall equation for this reaction is: Where, ka = [clo−][h +] [h clo] ≈ 3.0 ⋅ 10−8. Why hcl nacl is not a buffer? Web given in the question we have a combination of strong acid $hcl$ and a salt of strong acid and strong.
NaOH+HCl=NaCl+H2O balance it Brainly.in
Web for example, a buffer can be composed of dissolved acetic acid (hc2h3o2, a weak acid) and sodium acetate ( nac2h3o2). Web hcl and nacl are not buffer solutions. Where, ka = [clo−][h +] [h clo] ≈ 3.0 ⋅ 10−8. Web the combination of these two solutes would make a buffer solution. Hcl (g) + nacl (s) → nah (s).
Solved Write a balanced net ionic equation for the reaction
Is hcl+ nacl a complete reaction? Web hcl and nacl are not buffer solutions. Web may 6, 2018. Even though the second condition for a buffer solution is fulfilled, hcl as a strong. Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two.
Relative viscosity of DNA in TrisHCl/NaCl buffer solution (pH 7.2) in
Web if you dissolve nacl in water you will get some hcl molecules but there's definitely not going to be a significant concentration of hcl formed. Where, ka = [clo−][h +] [h clo] ≈ 3.0 ⋅ 10−8. Web for example, a buffer can be composed of dissolved acetic acid (hc2h3o2, a weak acid) and sodium acetate ( nac2h3o2). Hcl (g).
Mike's Online LabBook The Determination of the Mass of a Product of a
Mg + hcl > mgcl2 + h2 d. Cuo + h2 > cu + h2o c. A) hcl, nacl this contains. Web for example, a buffer can be composed of dissolved hc 2 h 3 o 2 (a weak acid) and nac 2 h 3 o 2 (the salt derived from that weak acid). If any molecules of $\ce{hcl}$ will.
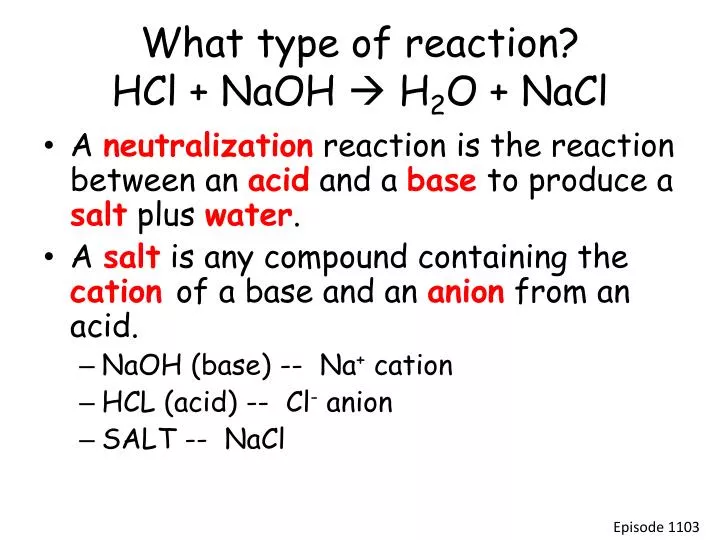
PPT What type of reaction? HCl + NaOH H 2 O + NaCl PowerPoint
Web the overall equation for this reaction is: Naoh + hcl → h2o and nacl. #1 a buffer is a mixture of a weak acid or weak base and its strong salt. Sehingga keduanya tidak memiliki pasangan. Moreover, consider the ionization of water,.
Best Would Hcl And Nacl Make A Buffer Home & Home
Sodium acetate is a salt that dissociates. Web the hcl + nacl reaction does not form a buffer solution because nacl is salt, and the acidity of hcl is high. Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the. Mg + hcl > mgcl2 + h2 d. Web we would like to show you a description.
Web The Hcl + Nacl Reaction Does Not Form A Buffer Solution Because Nacl Is Salt, And The Acidity Of Hcl Is High.
Hcn and nacn hcl and naoh nacn and naoh hcn and hcl hcl. Web for example, a buffer can be composed of dissolved acetic acid (hc2h3o2, a weak acid) and sodium acetate ( nac2h3o2). Na + h2o > naoh + h2 e. Web hcl and nacl are not buffer solutions.
Hcl (G) + Nacl (S) → Nah (S) + Cl 2 (G) A Salt (Nacl) And Water Are Produced In This Reaction When An Acid (Hcl) Reacts With A Base (Naoh),.
Web why is hcl and nacl not a buffer? Consider the buffer system's equilibrium, h clo ⇌ clo− + h +. Web the first condition for a buffer solution is not fulfilled. Web given in the question we have a combination of strong acid $hcl$ and a salt of strong acid and strong base $nacl(hcl + naoh)$ thus this combination cannot act as.
Sodium Acetate Is A Salt That Dissociates.
Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two. Is hcl+ nacl a complete reaction? Web we would like to show you a description here but the site won’t allow us. Mg + hcl > mgcl2 + h2 d.
Web The Combination Of These Two Solutes Would Make A Buffer Solution.
Web for example, a buffer can be composed of dissolved hc 2 h 3 o 2 (a weak acid) and nac 2 h 3 o 2 (the salt derived from that weak acid). If any molecules of $\ce{hcl}$ will form,. Naoh + hcl → h2o and nacl. Web may 6, 2018.