How Does Hydronium Ion Form
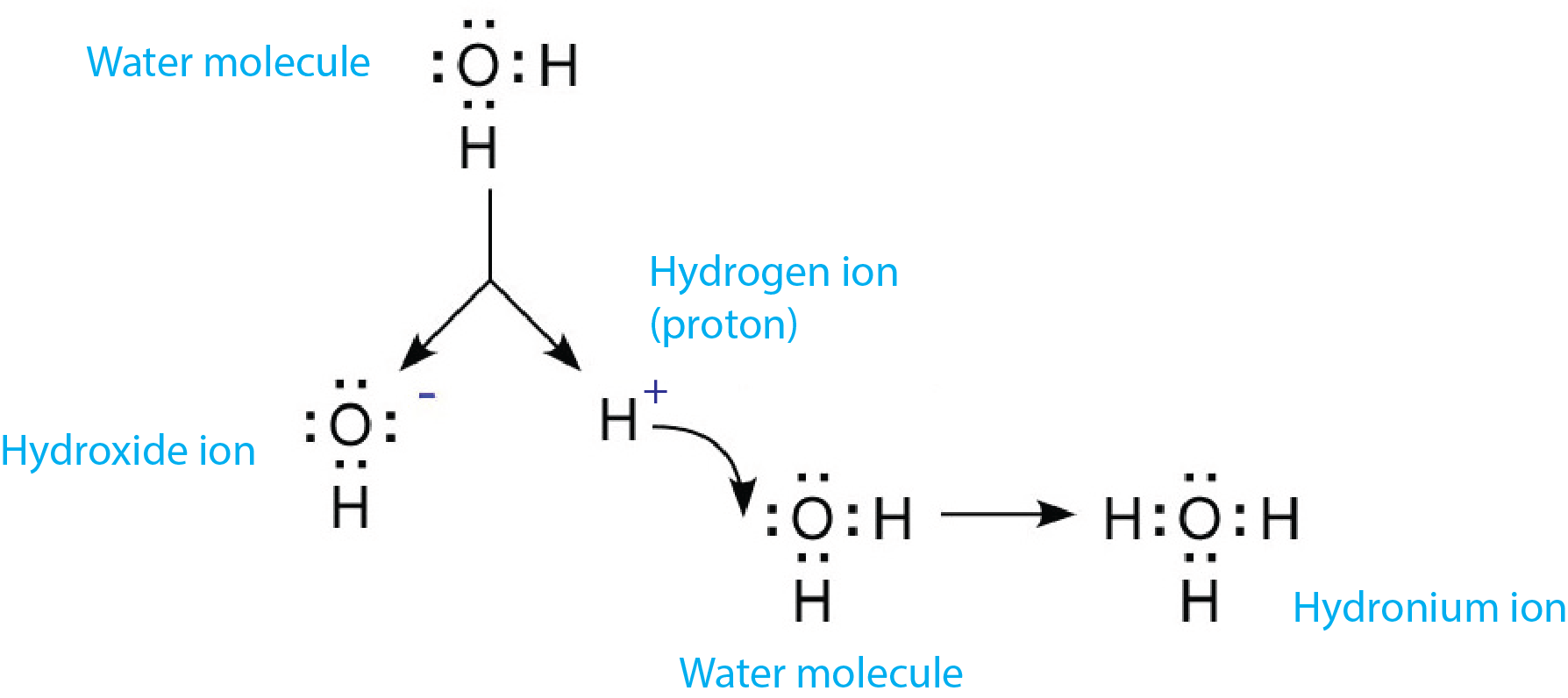
How Does Hydronium Ion Form - Web in aqueous solution of acids, acid dissociate to form hydrogen ions and combines with water molecules to form hydronium ion (h3o+). The concentration of h₃o⁺ in a strong acid solution is. Solution when a protic acid is added to water, it releases a hydrogen ion (h+) (also called aproton because a hydrogen atom missing one electron. It’s chemical formula is h3o+. Web it can be formed when an acid is present in water or simply in pure water. It can also be formed by the combination of a h+ ion with an h2o. Web hydronium ion definition, the hydrogen ion bonded to a molecule of water, h3o+, the form in which hydrogen ions are found in aqueous solution. Learn about the hydronium cation, which has the chemical formula h3o+. Web $\begingroup$ about the charge: Web how hydronium ion is formed?
It can also be formed by the combination of a h+ ion with an h2o. Solution when a protic acid is added to water, it releases a hydrogen ion (h+) (also called aproton because a hydrogen atom missing one electron. Web it can be formed when an acid is present in water or simply in pure water. Web $\begingroup$ about the charge: Chapter 5 / lesson 24. Thus, in aqueous solution only can a. If not, what information do you need in order to calculate it? Web how hydronium ion is formed? Web strong acids (such as hcl, hbr, hi, hno₃, hclo₄, and h₂so₄) ionize completely in water to produce hydronium ions. Web [1] in aqueous media, the free acid cation is mostly present as the hydrated proton (h 3 o) + but for convenience it is often referred to as a hydrogen ion or proton (h + ), and thus the.
It’s chemical formula is h3o+. Web the thing is an acid is any substance that increases the hydronium ion concentration of the solution or yields hydronium ions on dissociation. Web how hydronium ion is formed? It can also be formed by the combination of a h+ ion with an h2o. Hydronium is the simplest form of oxonium, which is any ion that. 0 + (+1) = +1, i.e. Water is uncharged, the hydrogen ion is charged, so the hydronium ion as the product of the two will be. Web in aqueous solution of acids, acid dissociate to form hydrogen ions and combines with water molecules to form hydronium ion (h3o+). Web it can be formed when an acid is present in water or simply in pure water. Thus, in aqueous solution only can a.
Hydronium Ion Definition & Formula Video & Lesson Transcript
Chapter 5 / lesson 24. The concentration of h₃o⁺ in a strong acid solution is. It’s chemical formula is h3o+. Hydronium is the simplest form of oxonium, which is any ion that. Water is uncharged, the hydrogen ion is charged, so the hydronium ion as the product of the two will be.
Hydronium Alchetron, The Free Social Encyclopedia
Web it can be formed when an acid is present in water or simply in pure water. Solution when a protic acid is added to water, it releases a hydrogen ion (h+) (also called aproton because a hydrogen atom missing one electron. Calculate the hydronium ion concentration of human blood. Web strong acids (such as hcl, hbr, hi, hno₃, hclo₄,.
H3O+, l'ion hydronium définition et explications
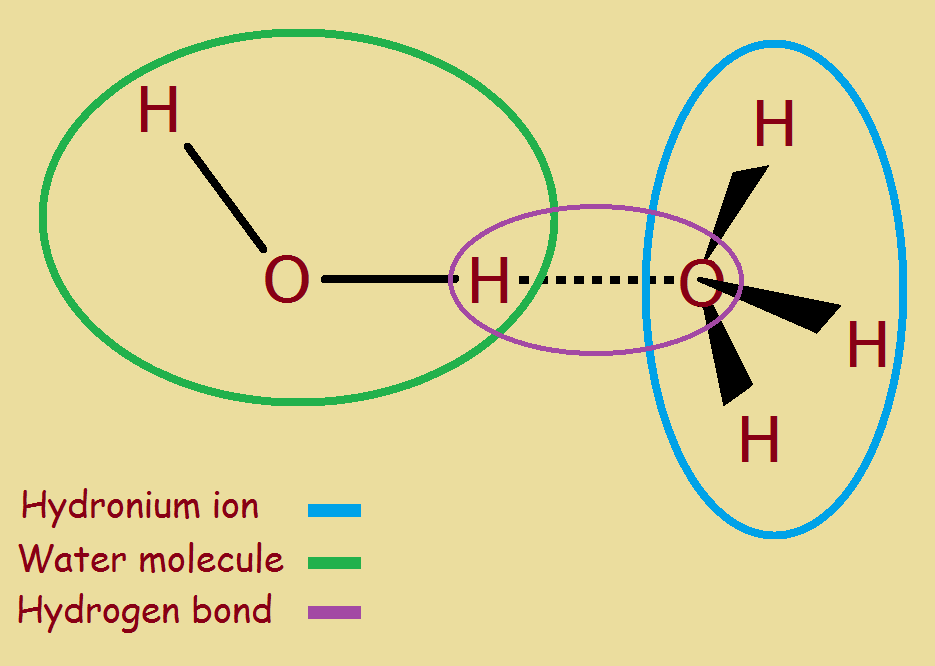
Web hydronium is what you get when you put water and hydrogen ions together, forming h 3 o +. It’s chemical formula is h3o+. Solution when a protic acid is added to water, it releases a hydrogen ion (h+) (also called aproton because a hydrogen atom missing one electron. Hydronium is the simplest form of oxonium, which is any ion.
Hydronium Ion Hydrate Its Cause and Molecular Structure
Learn about the hydronium cation, which has the chemical formula h3o+. The concentration of h₃o⁺ in a strong acid solution is. Web how hydronium ion is formed? Chapter 5 / lesson 24. Web $\begingroup$ about the charge:
Molecular Facts and Structures Chemical structure, Structure
0 + (+1) = +1, i.e. Chapter 5 / lesson 24. Web $\begingroup$ about the charge: Calculate the hydronium ion concentration of human blood. Hydronium is the key to calculating.
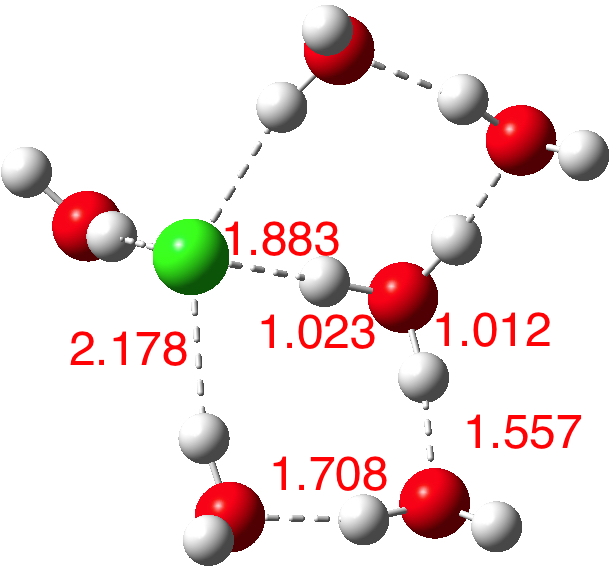
How many water molecules does it take to ionise HCl? « Henry Rzepa
Web [1] in aqueous media, the free acid cation is mostly present as the hydrated proton (h 3 o) + but for convenience it is often referred to as a hydrogen ion or proton (h + ), and thus the. Web in aqueous solution of acids, acid dissociate to form hydrogen ions and combines with water molecules to form hydronium.
Hydronium Ion Formation and Lewis Dot Structure YouTube
Chapter 5 / lesson 24. Web hydronium is what you get when you put water and hydrogen ions together, forming h 3 o +. Web $\begingroup$ about the charge: Web [1] in aqueous media, the free acid cation is mostly present as the hydrated proton (h 3 o) + but for convenience it is often referred to as a hydrogen.
Hydronium Ion Easy Science Easy science, Water molecule, Molecules
Web strong acids (such as hcl, hbr, hi, hno₃, hclo₄, and h₂so₄) ionize completely in water to produce hydronium ions. Web how hydronium ion is formed? Web the hydronium ion (h 3 o +), which is the hydrogen ion in aqueous solution, also belongs to this class. It’s chemical formula is h3o+. Web hydronium ion definition, the hydrogen ion bonded.
Multimedia pH and Color Change Chapter 6, Lesson 8 Middle School
Web hydronium ion definition, the hydrogen ion bonded to a molecule of water, h3o+, the form in which hydrogen ions are found in aqueous solution. If not, what information do you need in order to calculate it? Thus, in aqueous solution only can a. Web in aqueous solution of acids, acid dissociate to form hydrogen ions and combines with water.
Hydronium wikidoc
Web the hydronium ion (h 3 o +), which is the hydrogen ion in aqueous solution, also belongs to this class. Web strong acids (such as hcl, hbr, hi, hno₃, hclo₄, and h₂so₄) ionize completely in water to produce hydronium ions. Web $\begingroup$ about the charge: Web the thing is an acid is any substance that increases the hydronium ion.
0 + (+1) = +1, I.e.
Web hydronium is what you get when you put water and hydrogen ions together, forming h 3 o +. Web the thing is an acid is any substance that increases the hydronium ion concentration of the solution or yields hydronium ions on dissociation. Web how hydronium ion is formed? Water is uncharged, the hydrogen ion is charged, so the hydronium ion as the product of the two will be.
It Can Also Be Formed By The Combination Of A H+ Ion With An H2O.
Web in aqueous solution of acids, acid dissociate to form hydrogen ions and combines with water molecules to form hydronium ion (h3o+). Web hydronium ion definition, the hydrogen ion bonded to a molecule of water, h3o+, the form in which hydrogen ions are found in aqueous solution. If not, what information do you need in order to calculate it? Web hydrodium ions are the combination of a water molecule and a hydrogen ion, resulting in the formula, {eq}h_{3}o^{+} {/eq}.
Web $\Begingroup$ About The Charge:
Solution when a protic acid is added to water, it releases a hydrogen ion (h+) (also called aproton because a hydrogen atom missing one electron. The concentration of h₃o⁺ in a strong acid solution is. Hydronium is the key to calculating. Calculate the hydronium ion concentration of human blood.
Web The Hydronium Ion (H 3 O +), Which Is The Hydrogen Ion In Aqueous Solution, Also Belongs To This Class.
Hydronium is the simplest form of oxonium, which is any ion that. Web it can be formed when an acid is present in water or simply in pure water. Thus, in aqueous solution only can a. Learn about the hydronium cation, which has the chemical formula h3o+.