Why Does Water Form A Meniscus
Why Does Water Form A Meniscus - Web with water, you can think of it as when water sticks to the inside of a glass. Water forms a concave meniscus in a glass tube because the cohesive forces between water molecules are larger than the adhesive forces between water. A meniscus is the curved surface at the top of a column of liquid. A convex meniscus (sometimes called a. Web the meniscus's extracellular matrix (ecm) in a healthy knee is composed of 72% water; The remaining 28% is composed of organic matter and cells. A meniscal cyst is a. Web water on the knee is when fluid collects around and inside the knee joint, causing pain and swelling. 0 comments why a meniscus occurs adhesion is responsible for a meniscus. Web chemistry questions and answers.
0 comments why a meniscus occurs adhesion is responsible for a meniscus. Most liquids, including water, present a concave meniscus. A meniscus is the curved surface at the top of a column of liquid. Cohesion force of water molecules is greater. Also known as knee effusion or fluid on the knee, it can occur. The grainger college of engineering. | physics van | uiuc. Web water on the knee is when fluid collects around and inside the knee joint, causing pain and swelling. That is, the adhesive force between water and glass is greater than the cohesive force that exists. Web it is called the meniscus, but let me give you some more information on the subject.
Web the meniscus's extracellular matrix (ecm) in a healthy knee is composed of 72% water; In a science class, this. That is, the adhesive force between water and glass is greater than the cohesive force that exists. The grainger college of engineering. 75% of this organic matter is. Web why does water curve, and what is a meniscus? Web chemistry questions and answers. 1 point why does water forms a meniscus concave surface when put inside a capillary tube? Medically reviewed by oluseun olufade, md. Web it develops because of the surface tension of the liquid, which is defined as a property of liquid surface in which they behave in a cohesive and elastic manner.
Why some water droplets bounce on impact WordDisk
Why a meniscus occurs adhesion is responsible for a meniscus and this has to do in part with water’s fairly high surface. Adhesion is responsible for a meniscus and this has to do in. 1 point why does water forms a meniscus concave surface when put inside a capillary tube? Web the liquid appears to stick to the edge of.
How Meniscus Tears Affect Athletic Performance MASS4D® Foot Orthotics
That is, the adhesive force between water and glass is greater than the cohesive force that exists. Medically reviewed by oluseun olufade, md. A meniscus is the curved surface at the top of a column of liquid. 0 comments why a meniscus occurs adhesion is responsible for a meniscus. Most liquids, including water, present a concave meniscus.
Treating Meniscus Injuries in Adults Orthopaedic Associates of St
Web the strong adhesive forces between the water and the glass, pull the sides of the water upwards along the glass forming a concave shaped meniscus. Web it develops because of the surface tension of the liquid, which is defined as a property of liquid surface in which they behave in a cohesive and elastic manner. A meniscal cyst is.
Water Pouring Why does water stick to glass when pouring?
Web the liquid appears to stick to the edge of the container. Because water is a bit like a glue, water molecules like to attach. Web why does water form a meniscus? A meniscal cyst is a. The grainger college of engineering.
Chapter 11.3 Unique Properties of Liquids Chemwiki
| physics van | uiuc. Web eli5 why does water form a meniscus in a glass of water. Web the meniscus's extracellular matrix (ecm) in a healthy knee is composed of 72% water; Web with water, you can think of it as when water sticks to the inside of a glass. In a science class, this.
Explained !! Why Water Droplets are formed on Surface of cold bottle in
A convex meniscus (sometimes called a. Web the meniscus's extracellular matrix (ecm) in a healthy knee is composed of 72% water; That is, the adhesive force between water and glass is greater than the cohesive force that exists. Web updated on april 21, 2023. 75% of this organic matter is.
מדוע מים יוצרים קשרי מימן? מדע 2022
Web why does water curve, and what is a meniscus? Web updated on april 21, 2023. The grainger college of engineering. That is, the adhesive force between water and glass is greater than the cohesive force that exists. Most liquids, including water, present a concave meniscus.
Water Meniscus Differences Photograph by Andrew Lambert Photography
Most liquids, including water, present a concave meniscus. Adhesion is responsible for a meniscus and this has to do in. Web the liquid appears to stick to the edge of the container. | physics van | uiuc. 75% of this organic matter is.
Abbreviations and Acronyms for English Learners
Also known as knee effusion or fluid on the knee, it can occur. A meniscal cyst is a. Web the liquid appears to stick to the edge of the container. Web updated on april 21, 2023. Cohesion force of water molecules is greater.
meniscus measurement science mercury Fundamental Photographs The
Web why does water form a meniscus? This happens because water wets glass. Web it develops because of the surface tension of the liquid, which is defined as a property of liquid surface in which they behave in a cohesive and elastic manner. Cohesion force of water molecules is greater. Web chemistry questions and answers.
The Remaining 28% Is Composed Of Organic Matter And Cells.
Cohesion force of water molecules is greater. Most liquids, including water, present a concave meniscus. Web chemistry questions and answers. Web the liquid appears to stick to the edge of the container.
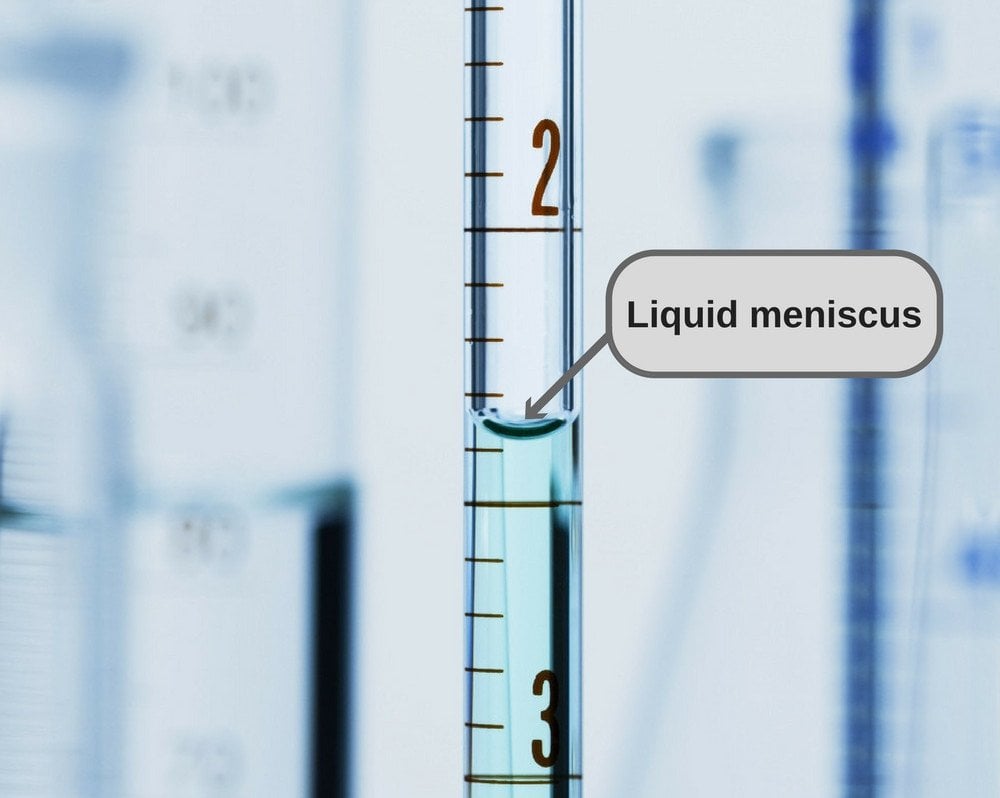
A Meniscus Is The Curved Surface At The Top Of A Column Of Liquid.
Web the strong adhesive forces between the water and the glass, pull the sides of the water upwards along the glass forming a concave shaped meniscus. A meniscal cyst is a. Also known as knee effusion or fluid on the knee, it can occur. 0 comments why a meniscus occurs adhesion is responsible for a meniscus.
Web The Meniscus's Extracellular Matrix (Ecm) In A Healthy Knee Is Composed Of 72% Water;
The grainger college of engineering. Web it develops because of the surface tension of the liquid, which is defined as a property of liquid surface in which they behave in a cohesive and elastic manner. A convex meniscus (sometimes called a. | physics van | uiuc.
Web It Is Called The Meniscus, But Let Me Give You Some More Information On The Subject.
Adhesion is responsible for a meniscus and this has to do in. 1 point why does water forms a meniscus concave surface when put inside a capillary tube? This happens because water wets glass. Water forms a concave meniscus in a glass tube because the cohesive forces between water molecules are larger than the adhesive forces between water.







:max_bytes(150000):strip_icc()/meniscus-curved-surface-meniscus-of-water-in-graduated-cylinder-liquid-volume-measured-by-reading-the-scale-at-the-bottom-of-the-meniscus-the-reading-is-82-6-ml-692027135-58dd0ca75f9b5846835e0e4e.jpg)
