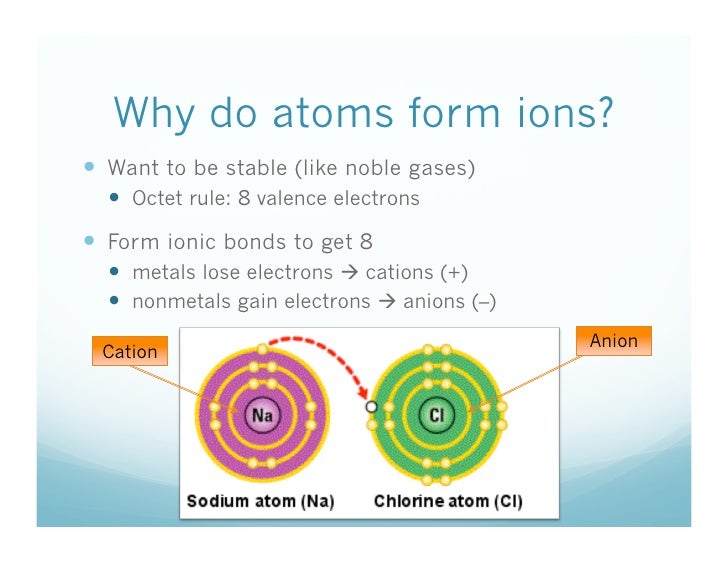
Why Do Nonmetals Tend To Form Anions
Why Do Nonmetals Tend To Form Anions - It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Web why do nonmetals and halogens tend to become anions? A neutral chlorine atom has seven electrons in its outermost shell. Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. Web why do nonmetal atoms form anions when they react to form compounds? Web (i) atomic radii decrease across the period from left to right, and (ii) metals tend to form cations, and (iii), in answer to your specific question, non metals tend to. Web chemistry question why do nonmetals tend to form anions rather than cations? Solution verified create an account to view solutions recommended textbook solutions. Web whether metals or non metals, the rule is that the element wants to reach the electron configuration of the nearest noble gas, the easiest way possible. Web nonmetals form negatively charged ions, or anions.
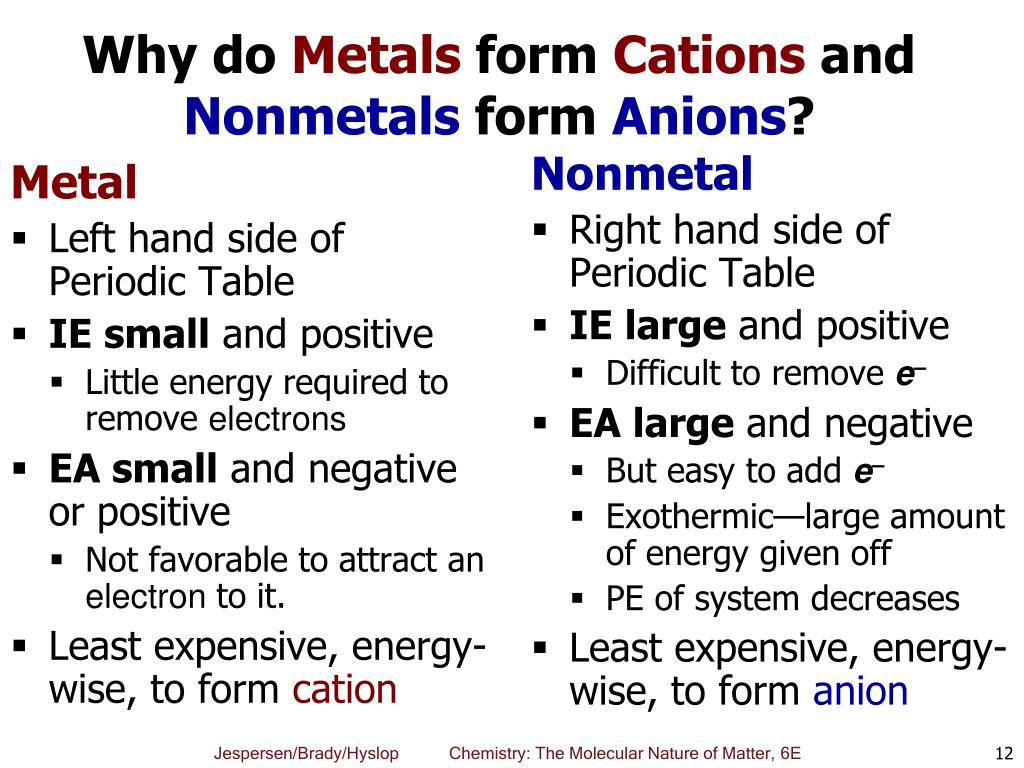
Web (i) atomic radii decrease across the period from left to right, and (ii) metals tend to form cations, and (iii), in answer to your specific question, non metals tend to. Web nonmetals form negatively charged ions, or anions. Solution verified create an account to view solutions recommended textbook solutions. Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound. Energy is released when electrons are removed from metal ions. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. A neutral chlorine atom has seven electrons in its outermost shell. These are electronegative elements with high ionization energies. Web view the full answer. Web whether metals or non metals, the rule is that the element wants to reach the electron configuration of the nearest noble gas, the easiest way possible.
Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. Web view the full answer. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Web most nonmetals become anions when they make ionic compounds. Aniseed may 11, 2020 may 11, 2020 #1 aniseed 8 0 tl;dr summary generally speaking the elements in. Web why do nonmetals and halogens tend to become anions? Web why do nonmetal atoms form anions when they react to form compounds? These are electronegative elements with high ionization energies. Solution verified create an account to view solutions recommended textbook solutions.
is incorreer? all s area eleseis erand e are metals b, all p area
These are electronegative elements with high ionization energies. Web why do nonmetals and halogens tend to become anions? Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. Energy is released when electrons are removed from metal ions. Web chemistry question why do nonmetals tend to form anions rather than cations?
Nonmetals on Periodic Table JulietatWaller
Web most nonmetals become anions when they make ionic compounds. Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. Web why do nonmetal atoms form anions when they react to form compounds? These are electronegative elements with high ionization energies. Aniseed may 11, 2020 may 11, 2020 #1 aniseed 8 0 tl;dr summary generally speaking the.
10 28 How Many Electrons Do Atoms Gain Lose
It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Web why do nonmetals and halogens tend to become anions? Web whether metals or non metals, the rule is that the element wants to reach the electron configuration of the nearest noble gas, the easiest way possible. Web 1 2 o2 +2h +.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
Energy is released when electrons are removed from metal ions. Web view the full answer. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web why do nonmetal atoms form anions when they react to form compounds? These are electronegative elements with high ionization energies.
Solved Question 6 (1 point) Why do metals tend to form
Solution verified create an account to view solutions recommended textbook solutions. Web whether metals or non metals, the rule is that the element wants to reach the electron configuration of the nearest noble gas, the easiest way possible. Energy is released when electrons are removed from metal ions. Web most nonmetals become anions when they make ionic compounds. Web (i).
Nonmetals and anion formation YouTube
Web (i) atomic radii decrease across the period from left to right, and (ii) metals tend to form cations, and (iii), in answer to your specific question, non metals tend to. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Aniseed may 11, 2020 may 11, 2020.
Solved Pls Answer This Question. Thanks. What's The Form...
Solution verified create an account to view solutions recommended textbook solutions. These are electronegative elements with high ionization energies. Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound. Web why do nonmetals and halogens tend to become anions? Web chemistry question why do nonmetals tend to form.
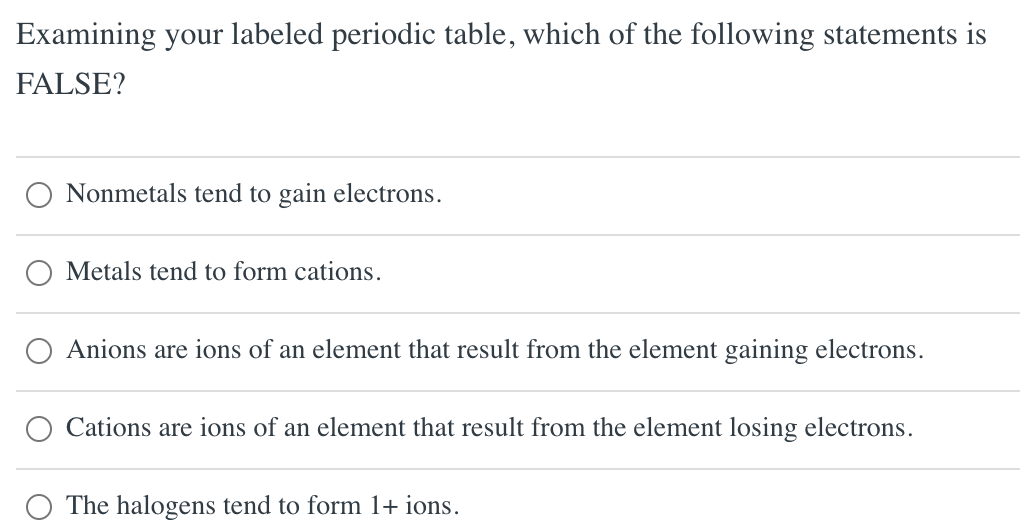
Solved Using your labeled periodic table, predict the charge
Web nonmetals form negatively charged ions, or anions. Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. Web why do nonmetal atoms form anions when they react to form compounds? Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web whether metals or.
10. List at least three examples of pairs... Physical Chemistry
Web why do nonmetals and halogens tend to become anions? Web chemistry question why do nonmetals tend to form anions rather than cations? Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web view the full answer. Web whether metals or non metals, the rule is that.
Halogens tend to form anions because A) losing
Web nonmetals form negatively charged ions, or anions. Web why do nonmetal atoms form anions when they react to form compounds? Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. Energy is released when.
Web View The Full Answer.
Web why do nonmetals and halogens tend to become anions? Web nonmetals form negatively charged ions, or anions. Aniseed may 11, 2020 may 11, 2020 #1 aniseed 8 0 tl;dr summary generally speaking the elements in. Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound.
Web Most Nonmetals Become Anions When They Make Ionic Compounds.
Web why do nonmetal atoms form anions when they react to form compounds? They do this because they need to gain one to three electrons in order to achieve an octet of valence electrons ,. These are electronegative elements with high ionization energies. Web (i) atomic radii decrease across the period from left to right, and (ii) metals tend to form cations, and (iii), in answer to your specific question, non metals tend to.
Web 1 2 O2 +2H + +2E− → H 2O ;E∘ = 1.23V.
Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web whether metals or non metals, the rule is that the element wants to reach the electron configuration of the nearest noble gas, the easiest way possible. Solution verified create an account to view solutions recommended textbook solutions. Energy is released when electrons are removed from metal ions.
Web Chemistry Question Why Do Nonmetals Tend To Form Anions Rather Than Cations?
A neutral chlorine atom has seven electrons in its outermost shell. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few.