Why Do Multiple Bonds Form
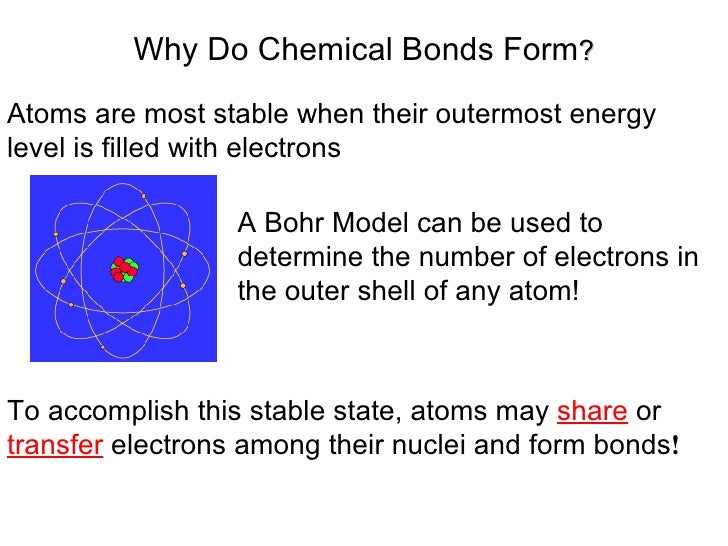
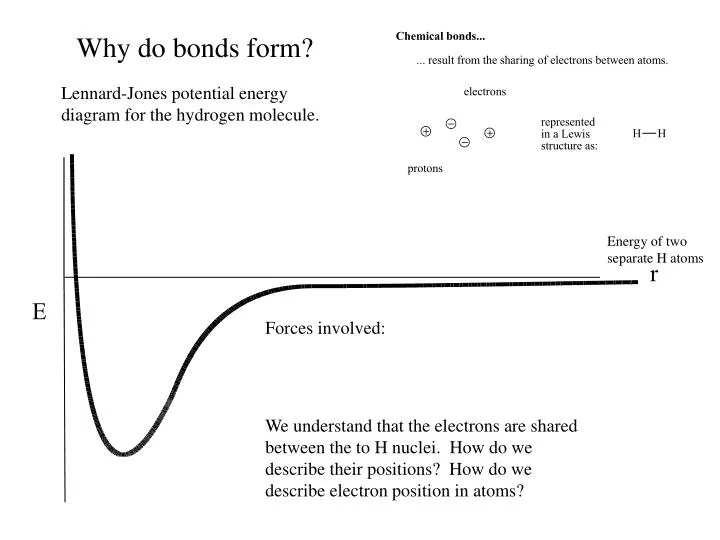
Why Do Multiple Bonds Form - Web covalent bonding is the sharing of one or more electron pairs. In order to fill these missing shells, multiple bonds form to fill these shells and as. So far, we have considered what are known as single bonds; Web in chemistry, the double bond rule states that elements with a principal quantum number greater than 2 for their valence electrons ( period 3 elements and higher) tend not to form. Web multiple bonds can form between two atoms. Sign this form in the presence of a. Web the number of bonds formed by an element can only be decided by the number of valence electrons participating in forming bonds. In many covalent bonding situations, multiple chemical bonds exist — more than one electron. Web why do multiple covalent bonds form? The σ bonds are usually formed by the overlap of hybridized.
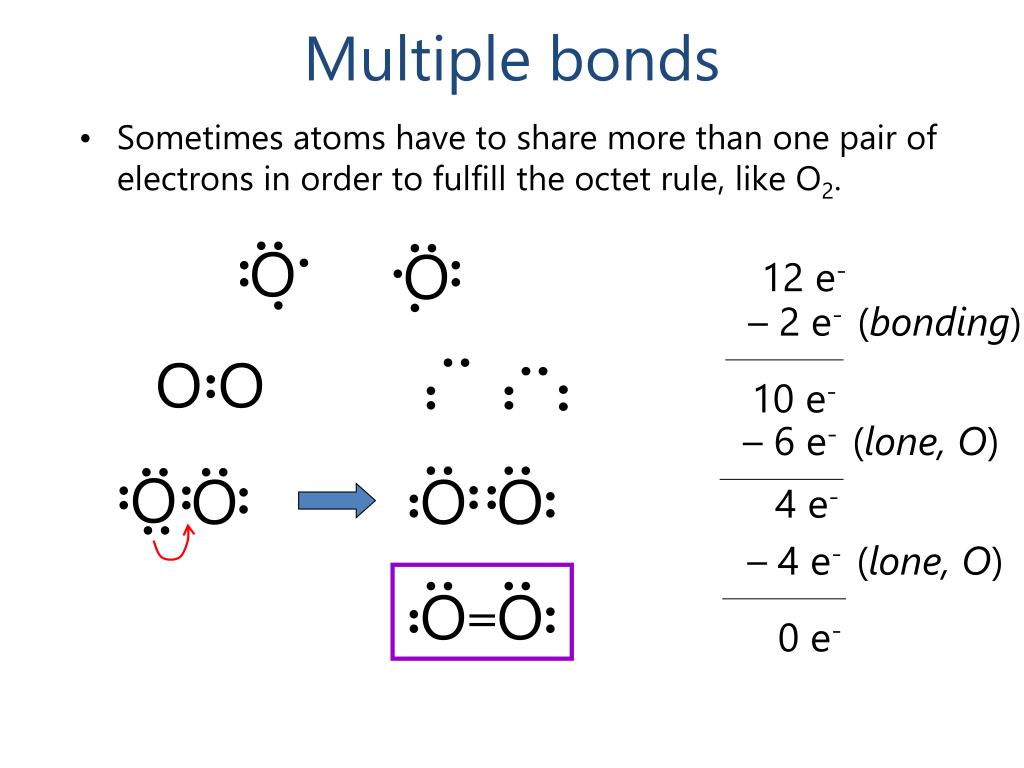
All of them are single pairing of electrons, but when the same atom forms multiple bonds. Web the reason for the formation of a multiple bond is an interaction of side to side type between the unhybridized p orbitals of the covalent bond constituent atoms. 4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy multiple covalent bonds form to attain. Web generally, the greater the risk, the higher the interest paid by a bond. A double bond is formed when two atoms use two electron pairs to form two covalent bonds; Web why do multiple covalent bonds form? So far, we have considered what are known as single bonds; Because there is a deficient amount of electrons in the orbitals. Returns on bonds are usually lower than those of stocks, but the. Special bond of indemnity by purchaser of united states savings bonds/notes involved in a chain letter scheme.
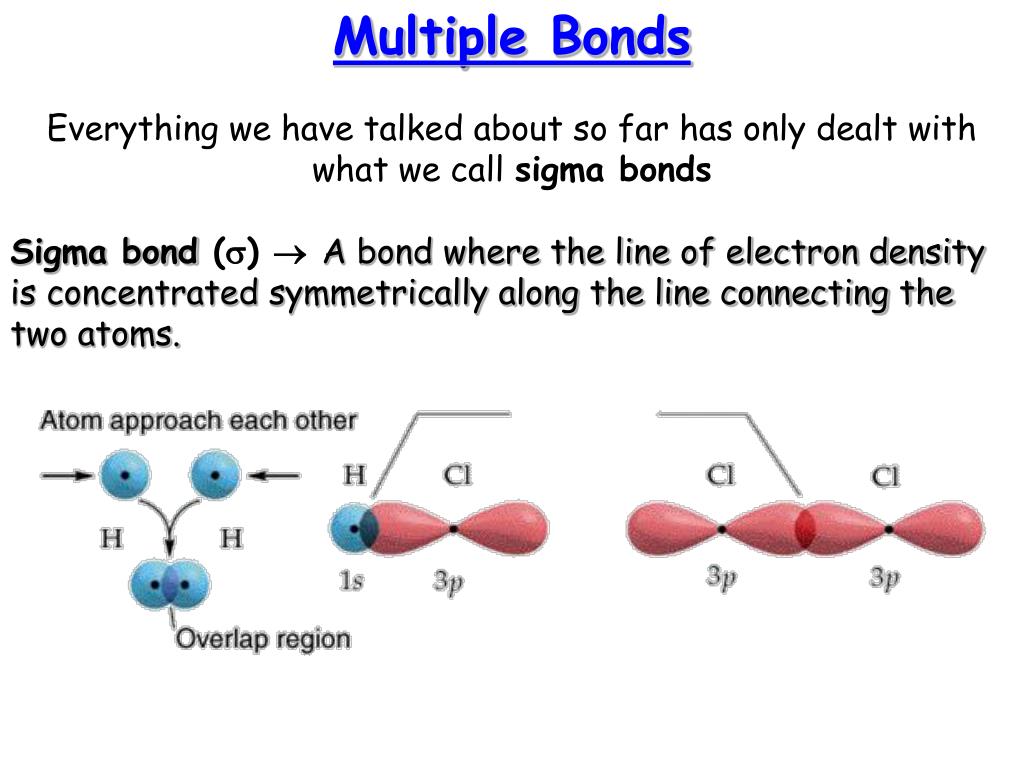
The σ bonds are usually formed by the overlap of hybridized. So far, we have considered what are known as single bonds; All bonds form as interactions of valence electrons of elements. Web generally, the greater the risk, the higher the interest paid by a bond. All of them are single pairing of electrons, but when the same atom forms multiple bonds. Returns on bonds are usually lower than those of stocks, but the. Atoms of different elements will form either one, two, three or four covalent. Web the number of bonds formed by an element can only be decided by the number of valence electrons participating in forming bonds. Web why do multiple covalent bonds form? Web covalent bonding is the sharing of one or more electron pairs.
Biochemistry Honors
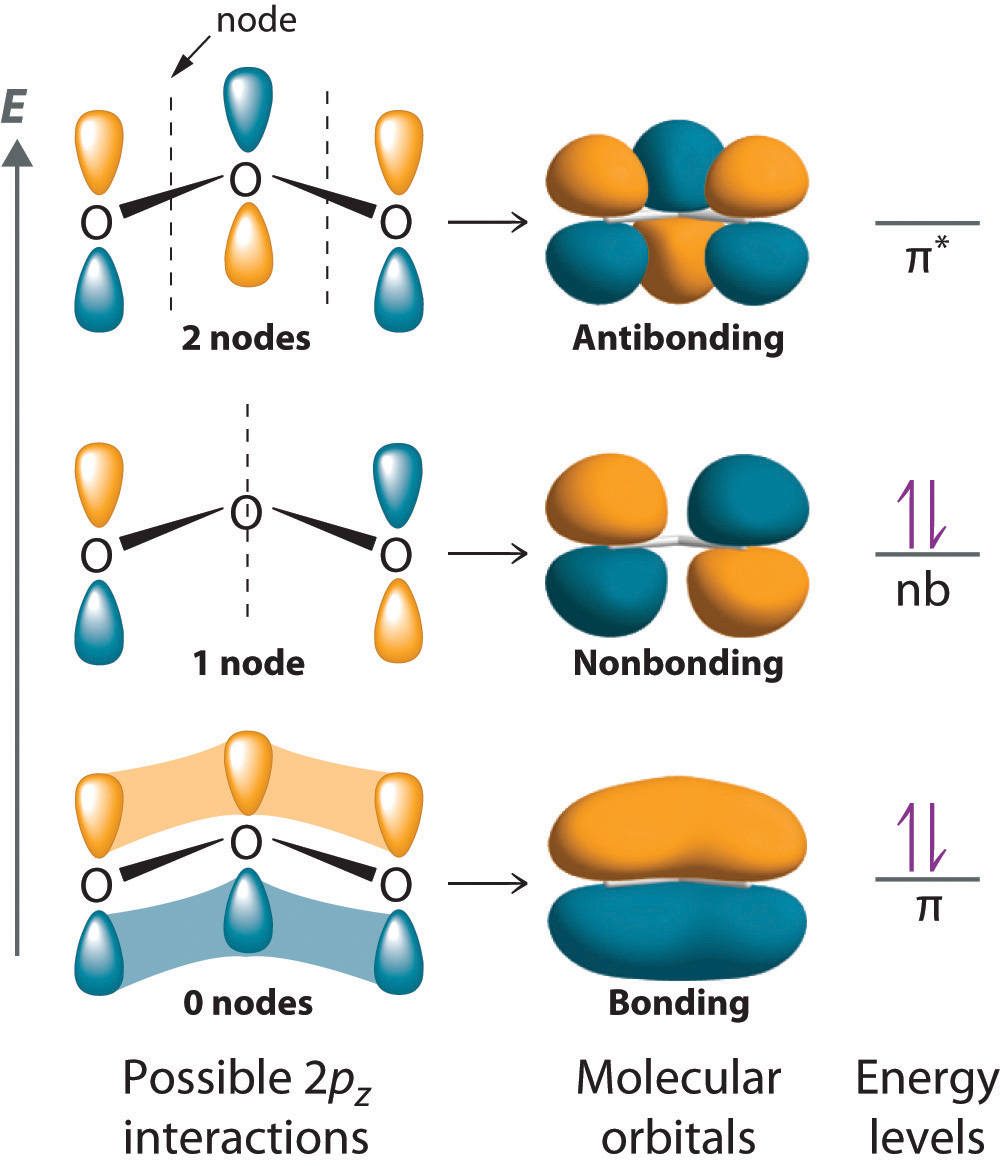
4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy multiple covalent bonds form to attain. Returns on bonds are usually lower than those of stocks, but the. In many covalent bonding situations, multiple chemical bonds exist — more than one electron. Web multiple bonds consist of a σ bond located along the axis between two.
PPT Base Pairing in DNA PowerPoint Presentation ID378280
Sign this form in the presence of a. So far, we have considered what are known as single bonds; Web.conditions, adjacent atoms will form multiple bonds with each other. Web covalent bonding is the sharing of one or more electron pairs. Special bond of indemnity by purchaser of united states savings bonds/notes involved in a chain letter scheme.
PPT Why do bonds form? PowerPoint Presentation, free download ID
Returns on bonds are usually lower than those of stocks, but the. Web in chemistry, the double bond rule states that elements with a principal quantum number greater than 2 for their valence electrons ( period 3 elements and higher) tend not to form. Web generally, the greater the risk, the higher the interest paid by a bond. All bonds.
Multiple Bonds — Double & Triple Bonds Expii
Web multiple bonds can form between two atoms. Atoms of different elements will form either one, two, three or four covalent. 4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy multiple covalent bonds form to attain. So far, we have considered what are known as single bonds; In many covalent bonding situations, multiple chemical bonds.
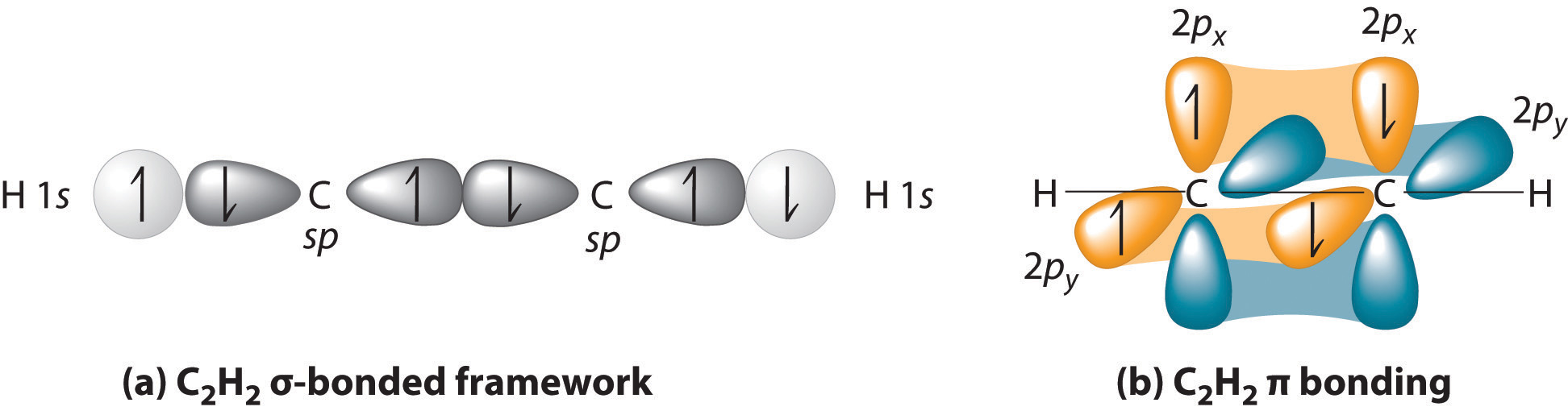
4.11 Multiple Bonds in MO Theory Chemistry LibreTexts
In many covalent bonding situations, multiple chemical bonds exist — more than one electron. Returns on bonds are usually lower than those of stocks, but the. Because there is a deficient amount of electrons in the orbitals. Web multiple bonds can form between two atoms. Web in chemistry, the double bond rule states that elements with a principal quantum number.
9.6 Multiple Bonds Chemistry LibreTexts
In order to fill these missing shells, multiple bonds form to fill these shells and as. 4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy multiple covalent bonds form to attain. Web why do multiple covalent bonds form? Sign this form in the presence of a. All bonds form as interactions of valence electrons of.
PPT Bonding PowerPoint Presentation, free download ID3050946
Because there is a deficient amount of electrons in the orbitals. The σ bonds are usually formed by the overlap of hybridized. Atoms of different elements will form either one, two, three or four covalent. Web covalent bonding is the sharing of one or more electron pairs. So far, we have considered what are known as single bonds;
PPT PowerPoint Presentation, free download ID2453814
In many covalent bonding situations, multiple chemical bonds exist — more than one electron. Web why do multiple covalent bonds form? The σ bonds are usually formed by the overlap of hybridized. Web generally, the greater the risk, the higher the interest paid by a bond. Special bond of indemnity by purchaser of united states savings bonds/notes involved in a.
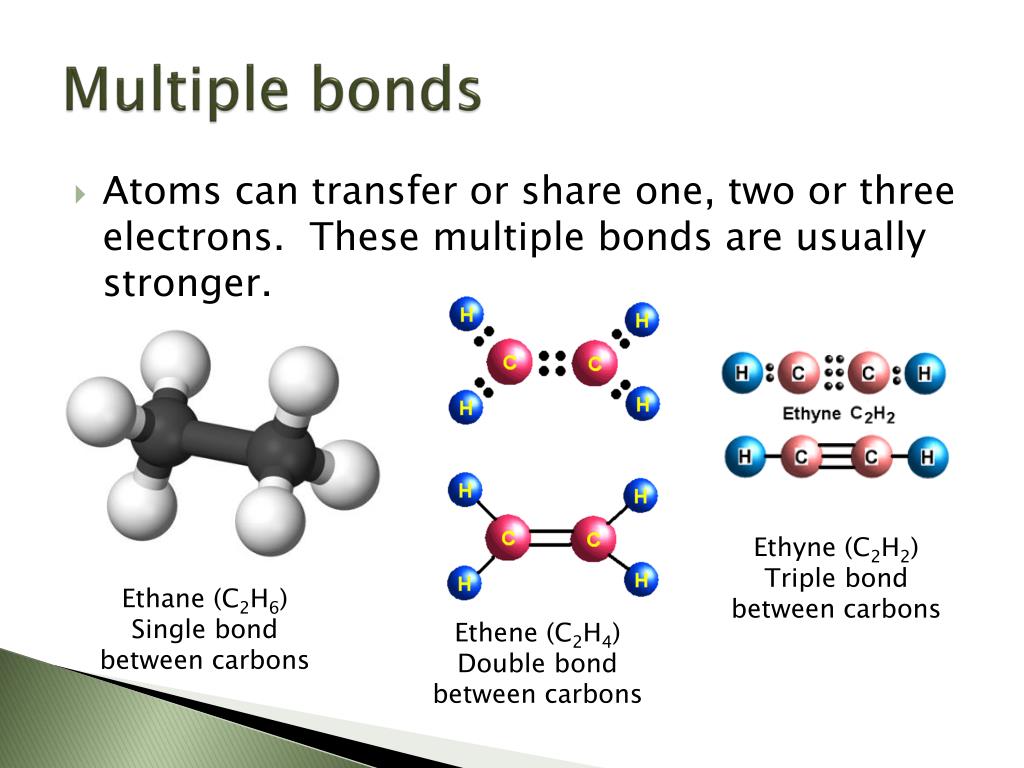
Single, Double, and Triple Bonds
All of them are single pairing of electrons, but when the same atom forms multiple bonds. In order to fill these missing shells, multiple bonds form to fill these shells and as. Because there is a deficient amount of electrons in the orbitals. Web the number of bonds formed by an element can only be decided by the number of.
Question Video Recalling the Type of Bond That Forms between
Web.conditions, adjacent atoms will form multiple bonds with each other. Double bonds form when the atoms share two pairs of electrons, and triple bonds form when they share three pairs. Web why do multiple covalent bonds form? Web multiple bonds can form between two atoms. In order to fill these missing shells, multiple bonds form to fill these shells and.
Special Bond Of Indemnity By Purchaser Of United States Savings Bonds/Notes Involved In A Chain Letter Scheme.
The σ bonds are usually formed by the overlap of hybridized. Atoms of different elements will form either one, two, three or four covalent. Web the reason for the formation of a multiple bond is an interaction of side to side type between the unhybridized p orbitals of the covalent bond constituent atoms. In many covalent bonding situations, multiple chemical bonds exist — more than one electron.
In Order To Fill These Missing Shells, Multiple Bonds Form To Fill These Shells And As.
So far, we have considered what are known as single bonds; Web the number of bonds formed by an element can only be decided by the number of valence electrons participating in forming bonds. Double bonds form when the atoms share two pairs of electrons, and triple bonds form when they share three pairs. 4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy multiple covalent bonds form to attain.
Sign This Form In The Presence Of A.
Web.conditions, adjacent atoms will form multiple bonds with each other. Web covalent bonding is the sharing of one or more electron pairs. Web in chemistry, the double bond rule states that elements with a principal quantum number greater than 2 for their valence electrons ( period 3 elements and higher) tend not to form. All of them are single pairing of electrons, but when the same atom forms multiple bonds.
All Bonds Form As Interactions Of Valence Electrons Of Elements.
A double bond is formed when two atoms use two electron pairs to form two covalent bonds; Web why do multiple covalent bonds form? Web multiple bonds can form between two atoms. Web multiple bonds consist of a σ bond located along the axis between two atoms and one or two π bonds.