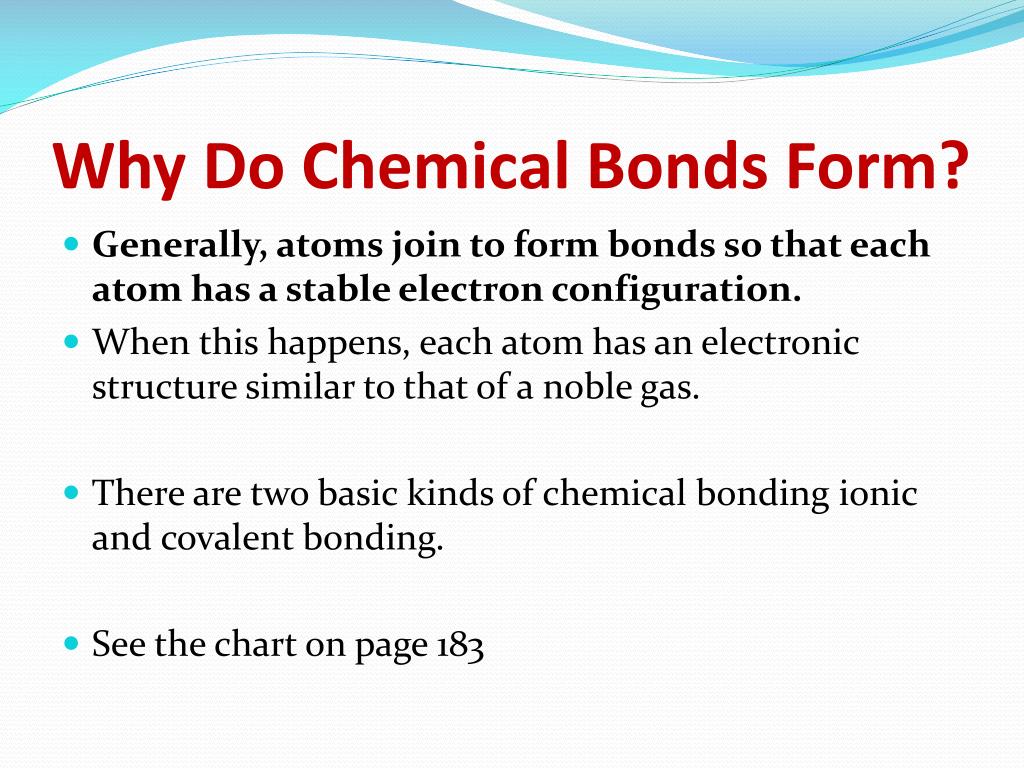
Why Do Chemical Bonds Form Between Atoms
Why Do Chemical Bonds Form Between Atoms - Atoms can form several types of chemical bonds. The combination of multiple atoms, or chemical bonding, forms molecules. So, how do atoms make friends? High (less stable) as independent particles, most. For example, an oxygen atom can bond with. (3) of different atoms that bonds the atoms together. The atom that gains electrons has an overall negative. These chemical bonds further bond with other atoms and/or molecules to form chemical compounds. Web why do chemical bonds form between atoms? Web nonpolar covalent bonds form between two atoms of the same element or between different elements that share the electrons equally.
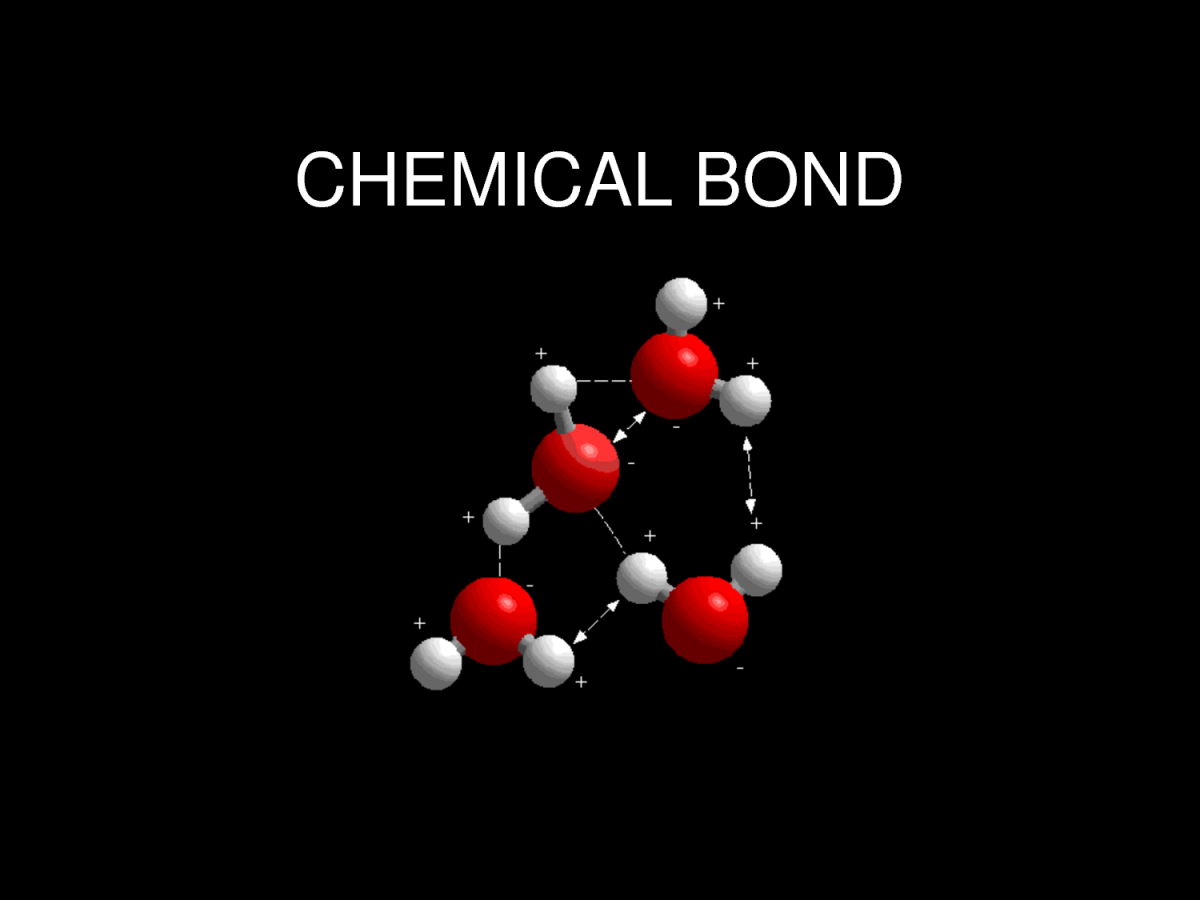
Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. Web when atoms get together to make friends, it is called chemical bonding. For example, an oxygen atom can bond with. Web a chemical bond is a.electrical attraction between the.nuclei and the. Web the attraction between atoms results into a chemical bond. The combination of multiple atoms, or chemical bonding, forms molecules. High (less stable) as independent particles, most. Atoms can form several types of chemical bonds. The atom that gains electrons has an overall negative. Web nonpolar covalent bonds form between two atoms of the same element or between different elements that share the electrons equally.
Web a chemical bond is a.electrical attraction between the.nuclei and the. Atomic structure and bonding finally, in some materials each atom gives up an outer electron that then floats freely—in essence, the electron is shared by all of. Chemical bonds are formed when electrons in different atoms. Web ionic bonds form between two atoms when one or more electrons are completely transferred from one atom to the other. Atoms can form several types of chemical bonds. High (less stable) as independent particles, most. The simplest answer is with their electrons and, more specifically, with. The atom that gains electrons has an overall negative. These bonds are interactions between two atoms that hold the atoms together. So, how do atoms make friends?
PPT COMPOUNDS PowerPoint Presentation, free download ID6094192
The simplest answer is with their electrons and, more specifically, with. Web nonpolar covalent bonds form between two atoms of the same element or between different elements that share the electrons equally. The atom that gains electrons has an overall negative. Web why do chemical bonds form between atoms? Web atoms can join together by forming a chemical bond, which.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
High (less stable) as independent particles, most. Web when atoms get together to make friends, it is called chemical bonding. Atomic structure and bonding finally, in some materials each atom gives up an outer electron that then floats freely—in essence, the electron is shared by all of. Atoms can form several types of chemical bonds. Web atoms can join together.
What Are Chemical Bonds and Why Do They Form?
So, how do atoms make friends? Atoms can form several types of chemical bonds. The simplest answer is with their electrons and, more specifically, with. (3) of different atoms that bonds the atoms together. The combination of multiple atoms, or chemical bonding, forms molecules.
Atoms and chemical bonds YouTube
High (less stable) as independent particles, most. Web why do chemical bonds form between atoms? Web a chemical bond is a.electrical attraction between the.nuclei and the. Chemical bonds are formed when electrons in different atoms. The simplest answer is with their electrons and, more specifically, with.
PPT The Structure of Matter Chapter 6 PowerPoint Presentation, free
(3) of different atoms that bonds the atoms together. Web nonpolar covalent bonds form between two atoms of the same element or between different elements that share the electrons equally. The atom that gains electrons has an overall negative. These bonds are interactions between two atoms that hold the atoms together. Chemical bonds are formed when electrons in different atoms.
Why Do Atoms Form Chemical Bond ,By chemistry with concept. YouTube
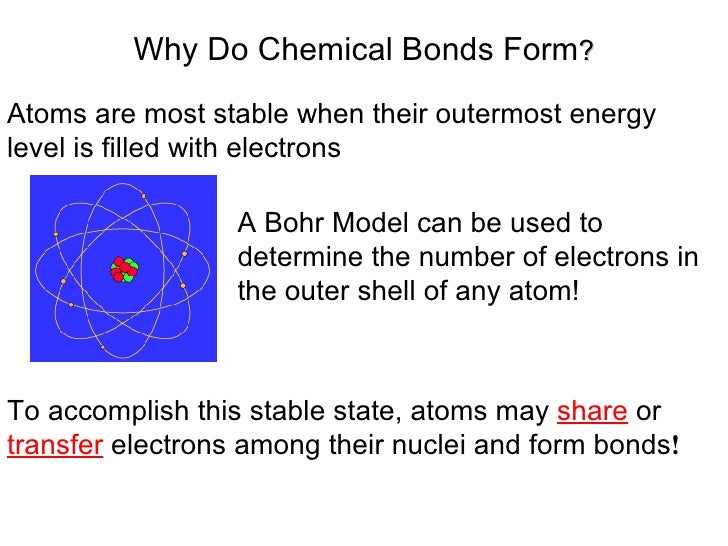
The combination of multiple atoms, or chemical bonding, forms molecules. These chemical bonds further bond with other atoms and/or molecules to form chemical compounds. Web atoms are individual units made up of protons, neutrons, and electrons. Atomic structure and bonding finally, in some materials each atom gives up an outer electron that then floats freely—in essence, the electron is shared.
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind
Atoms can form several types of chemical bonds. Web the attraction between atoms results into a chemical bond. Web when atoms get together to make friends, it is called chemical bonding. So, how do atoms make friends? Web why do chemical bonds form between atoms?
2.2 Chemical Bonds Anatomy & Physiology
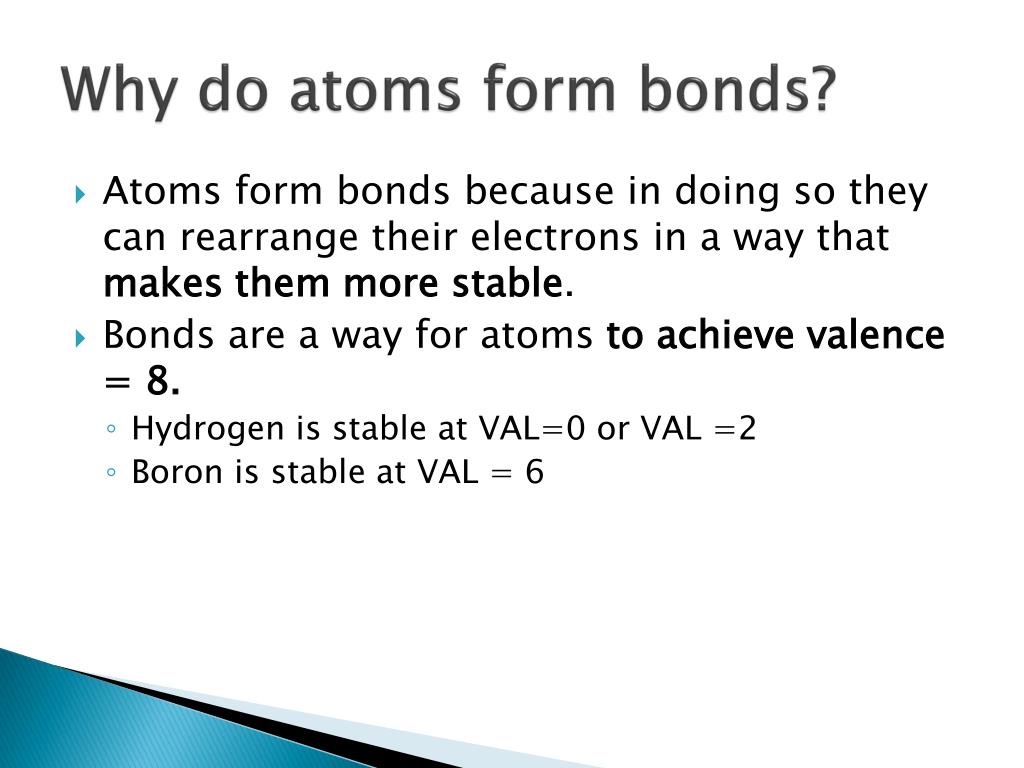
The combination of multiple atoms, or chemical bonding, forms molecules. Atoms will transfer, accept, or share electrons necessary to attain a complete outer valence shell containing 8 electrons. These bonds are interactions between two atoms that hold the atoms together. Web the attraction between atoms results into a chemical bond. The atom that gains electrons has an overall negative.
Biochemistry Honors
Web atoms are individual units made up of protons, neutrons, and electrons. Web ionic bonds form between two atoms when one or more electrons are completely transferred from one atom to the other. Web when atoms get together to make friends, it is called chemical bonding. Atomic structure and bonding finally, in some materials each atom gives up an outer.
PPT Chemical Bonding What and Why PowerPoint Presentation, free
Web a chemical bond is a.electrical attraction between the.nuclei and the. Web nonpolar covalent bonds form between two atoms of the same element or between different elements that share the electrons equally. These bonds are interactions between two atoms that hold the atoms together. Web ionic bonds form between two atoms when one or more electrons are completely transferred from.
Web The Attraction Between Atoms Results Into A Chemical Bond.
For example, an oxygen atom can bond with. Web atoms are individual units made up of protons, neutrons, and electrons. Atoms can form several types of chemical bonds. Web a chemical bond is a.electrical attraction between the.nuclei and the.
Web Why Do Chemical Bonds Form Between Atoms?
There are several types of bond. Web ionic bonds form between two atoms when one or more electrons are completely transferred from one atom to the other. These bonds are interactions between two atoms that hold the atoms together. The atom that gains electrons has an overall negative.
Atomic Structure And Bonding Finally, In Some Materials Each Atom Gives Up An Outer Electron That Then Floats Freely—In Essence, The Electron Is Shared By All Of.
Atoms will transfer, accept, or share electrons necessary to attain a complete outer valence shell containing 8 electrons. So, how do atoms make friends? The combination of multiple atoms, or chemical bonding, forms molecules. The simplest answer is with their electrons and, more specifically, with.
Chemical Bonds Are Formed When Electrons In Different Atoms.
Web nonpolar covalent bonds form between two atoms of the same element or between different elements that share the electrons equally. High (less stable) as independent particles, most. (3) of different atoms that bonds the atoms together. These chemical bonds further bond with other atoms and/or molecules to form chemical compounds.