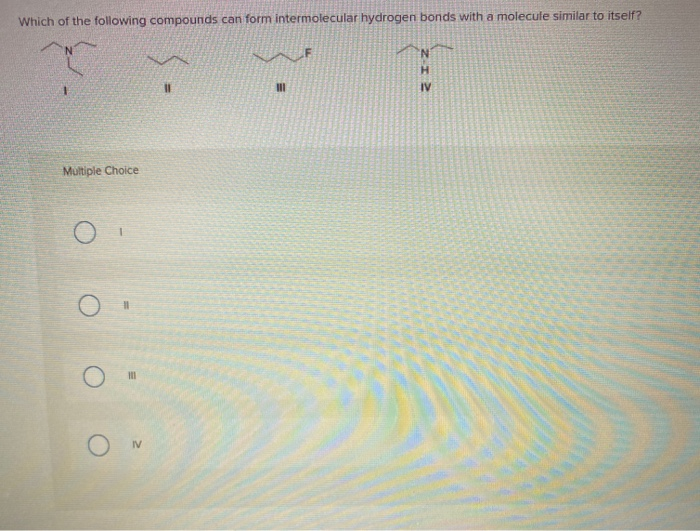
Which Of The Following Can Form Intermolecular Hydrogen Bonds
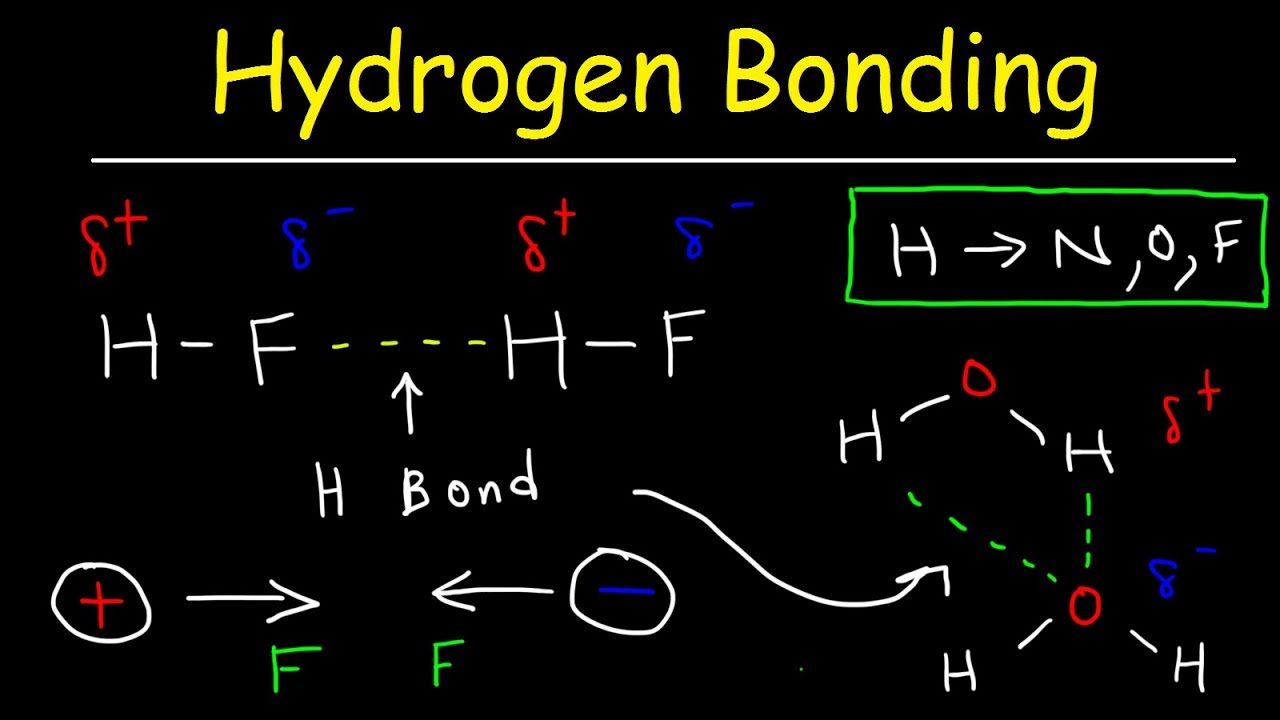
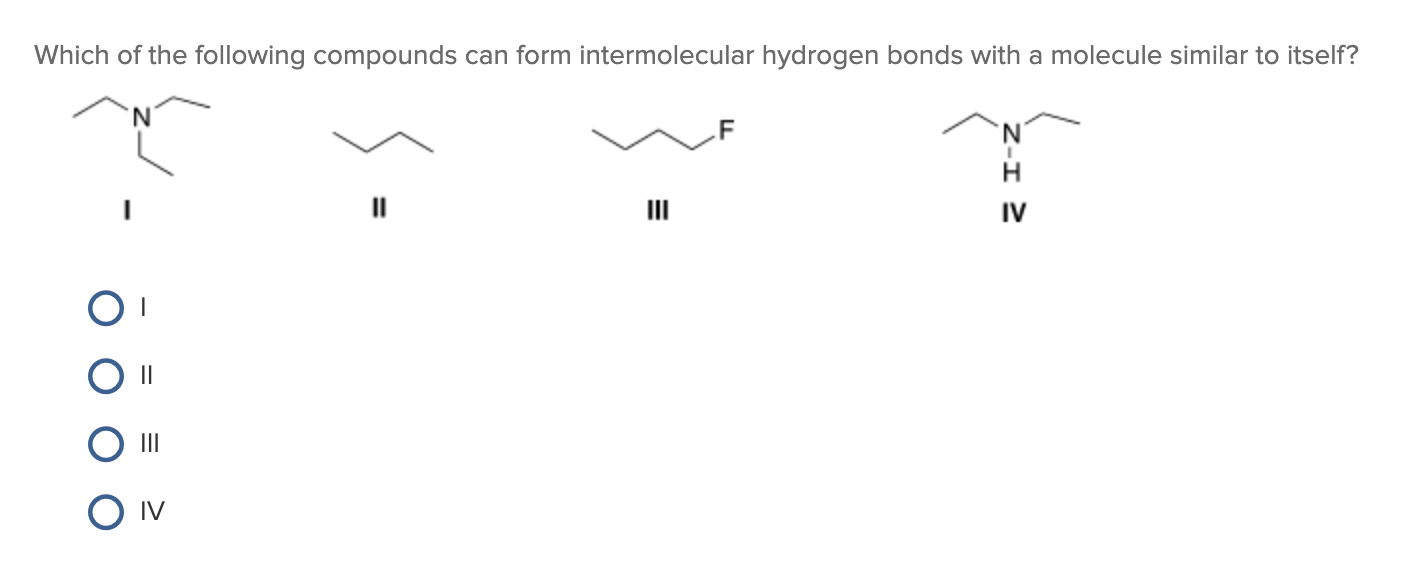
Which Of The Following Can Form Intermolecular Hydrogen Bonds - Which of the following compounds can form intermolecular hydrogen bonds?. Web a hydrogen bond is an electrostatic attraction between a partially negative n or o atom and a partially positive hydrogen atom that is covalently bound to a different n or o atom. Web which of the following compounds can form intermolecular hydrogen bonds? Hydrogen bonds have strengths ranging from 5 kj/mol to 50 kj/mol. Web intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °c) compared to the other group 16 hydrides, which have little capability to hydrogen bond. Web class 11 >> chemistry >> chemical bonding and molecular structure >> hydrogen bonding >> which of the following compounds can for question which of the. What types of intermolecular forces exist in a sample of acetone? Those bonds are represented between hydrogen and ah hah login, which is x, the halogen. Web science chemistry chemistry questions and answers which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron. Hydrogen bonding is the strongest type of.
Which of the following compounds can form intermolecular hydrogen bonds? Web which of the following compounds can form intermolecular hydrogen bonds? (select all that apply.) hf br2 ch3oh ch4 In summary, hydrogen bonds are. E) all of these compounds can form hydrogen bonds. Those bonds are represented between hydrogen and ah hah login, which is x, the halogen. Web this type of intermolecular bond is stronger than london dispersion forces with the same number of electrons. Hydrogen bonds have strengths ranging from 5 kj/mol to 50 kj/mol. Web hydrogen bonding in organic molecules containing nitrogen. Web intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °c) compared to the other group 16 hydrides, which have little capability to hydrogen bond.
Hydrogen bonding is the strongest type of. (select all that apply.) hf br2 ch3oh ch4 Which of the following compounds can form intermolecular hydrogen bonds? Some compounds can form into molecular hydrogen bonds. Web science chemistry chemistry questions and answers which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron. Hydrogen bonds have strengths ranging from 5 kj/mol to 50 kj/mol. Web a hydrogen bond is an electrostatic attraction between a partially negative n or o atom and a partially positive hydrogen atom that is covalently bound to a different n or o atom. Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. What types of intermolecular forces exist in a sample of acetone?
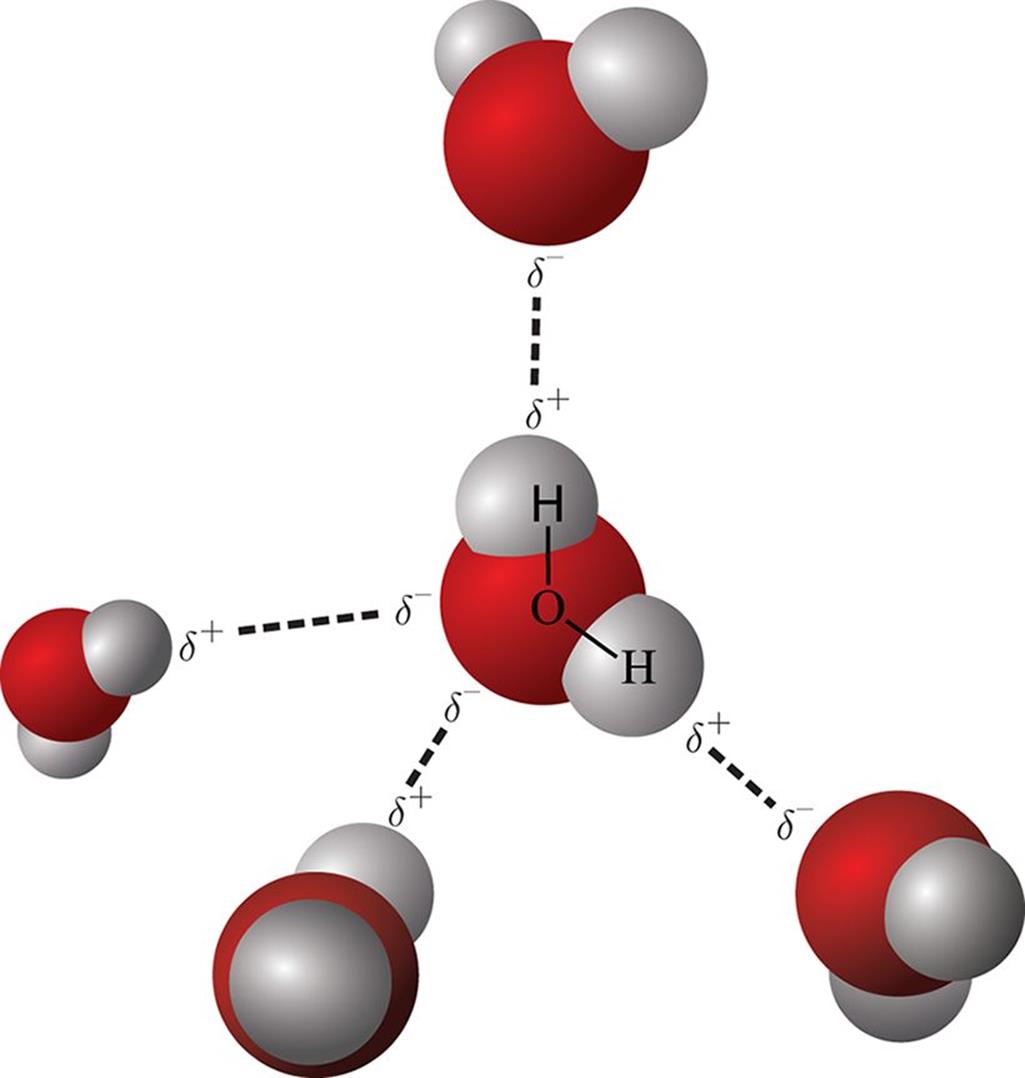
Diagram Of Water Molecules Hydrogen Bonding
Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? Hydrogen bonding is the strongest type of. Those bonds are represented between hydrogen and ah hah login, which is x, the halogen. Some compounds can form into molecular hydrogen bonds. Web you'll get a detailed solution from a subject matter expert that helps you learn.
Solved Which of the following compounds can form
What types of intermolecular forces exist in a sample of acetone? Web science chemistry chemistry questions and answers which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron. (select all that apply.) hf br2 ch3oh ch4 Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? Those bonds.
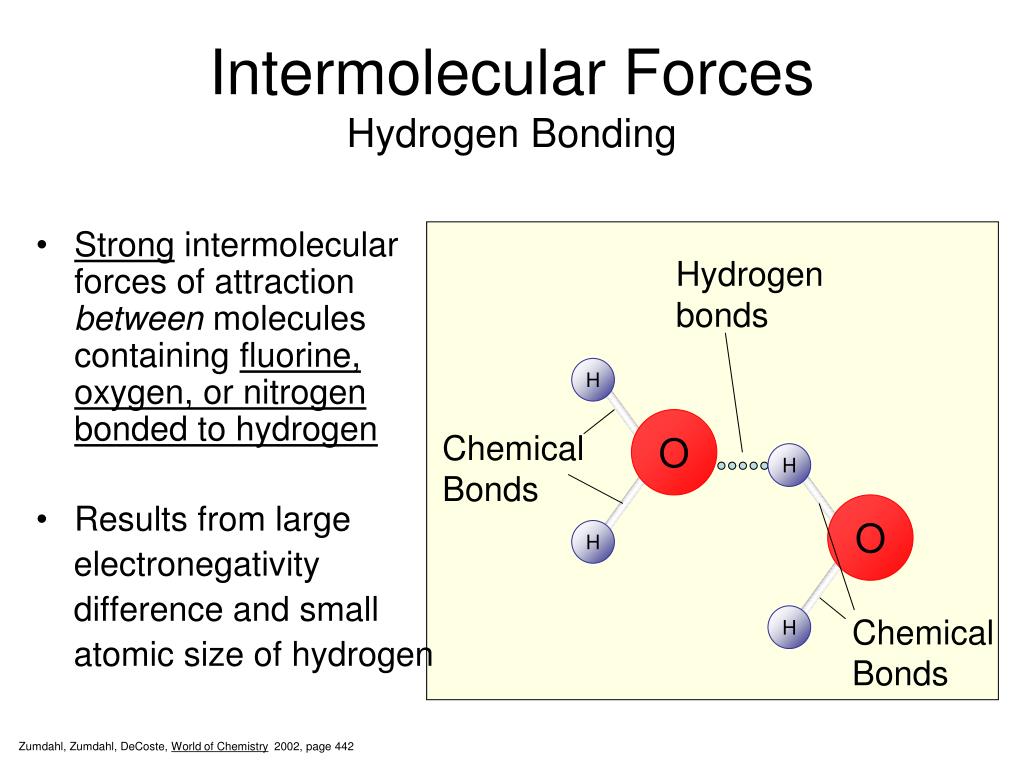
Figure 3.15. Hydrogen Bonding in Water
E) all of these compounds can form hydrogen bonds. Web which of the following compounds can form intermolecular hydrogen bonds? Which of the following compounds can form intermolecular hydrogen bonds? Hydrogen bonding is the strongest type of. Hydrogen bonds have strengths ranging from 5 kj/mol to 50 kj/mol.
Solved Which of the following compounds can form
Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? (select all that apply.) hf br2 ch3oh ch4 What types of intermolecular forces exist in a sample of acetone? Those bonds are represented between hydrogen and ah hah login, which is x, the halogen. Web this type of intermolecular bond is stronger than london dispersion.
Difference Between Intermolecular and Intramolecular Hydrogen Bonding
Web which of the following compounds can form intermolecular hydrogen bonds? Which of the following compounds can form intermolecular hydrogen bonds?. Web science chemistry chemistry questions and answers which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron. In summary, hydrogen bonds are. Hydrogen bonding is the strongest type of.
PPT Intermolecular Forces PowerPoint Presentation ID705859
Web intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °c) compared to the other group 16 hydrides, which have little capability to hydrogen bond. Web a hydrogen bond is an electrostatic attraction between a partially negative n or o atom and a partially positive hydrogen atom that is covalently bound to a different n or.
Solved Which Of The Following Compounds Can Form Intermol...
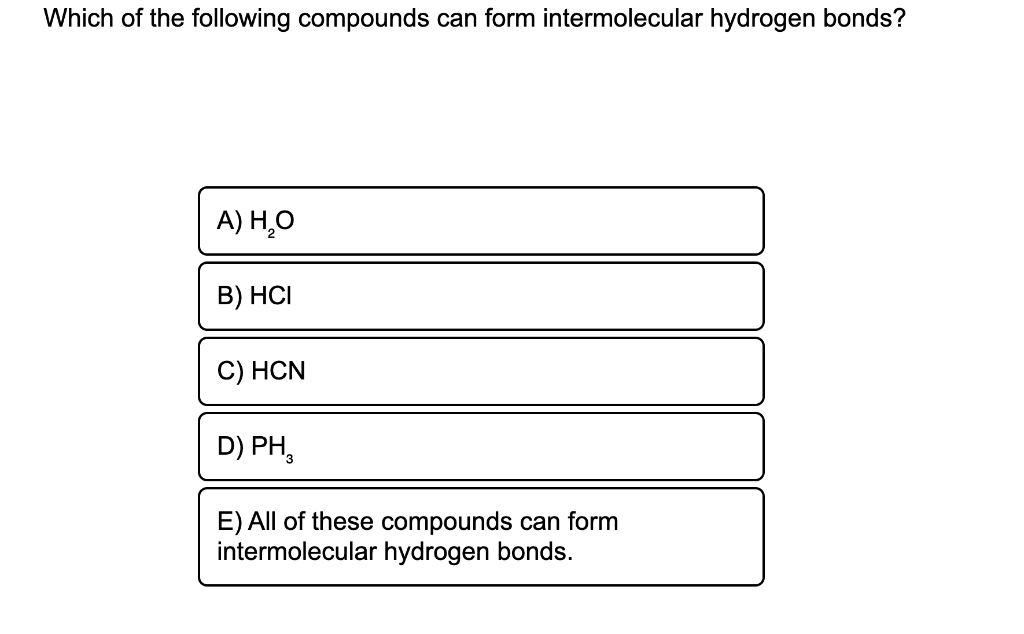
In summary, hydrogen bonds are. A) h2o b) hcl c) hcn d) ph3 e) all of these can form intermoleculat hydrogen bonds E) all of these compounds can form hydrogen bonds. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web intermolecular hydrogen bonding is responsible for the high boiling point of.
IGCSE Physical and Chemical Properties of Hydrocarbons IGCSE And IAL
(select all that apply.) hf br2 ch3oh ch4 Web class 11 >> chemistry >> chemical bonding and molecular structure >> hydrogen bonding >> which of the following compounds can for question which of the. Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? In summary, hydrogen bonds are. Web science chemistry chemistry questions and.
Image result for intermolecular forces
Web a hydrogen bond is an electrostatic attraction between a partially negative n or o atom and a partially positive hydrogen atom that is covalently bound to a different n or o atom. Web this type of intermolecular bond is stronger than london dispersion forces with the same number of electrons. Web hydrogen bonding in organic molecules containing nitrogen. Web.
Hydrogen Bonding in Ethanol (C2H5OH) YouTube
Which of the following compounds can form intermolecular hydrogen bonds? In summary, hydrogen bonds are. E) all of these compounds can form hydrogen bonds. Web class 11 >> chemistry >> chemical bonding and molecular structure >> hydrogen bonding >> which of the following compounds can for question which of the. Web science chemistry chemistry questions and answers which of the.
Hydrogen Bonding Is The Strongest Type Of.
What types of intermolecular forces exist in a sample of acetone? Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °c) compared to the other group 16 hydrides, which have little capability to hydrogen bond. A) h2o b) hcl c) hcn d) ph3 e) all of these can form intermoleculat hydrogen bonds
Some Compounds Can Form Into Molecular Hydrogen Bonds.
(select all that apply.) hf br2 ch3oh ch4 Those bonds are represented between hydrogen and ah hah login, which is x, the halogen. Which of the following compounds can form intermolecular hydrogen bonds? Web class 11 >> chemistry >> chemical bonding and molecular structure >> hydrogen bonding >> which of the following compounds can for question which of the.
Web Hydrogen Bonding In Organic Molecules Containing Nitrogen.
Web which of the following compounds can form intermolecular hydrogen bonds? Web science chemistry chemistry questions and answers which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron. E) all of these compounds can form hydrogen bonds. In summary, hydrogen bonds are.
Which Of The Following Compounds Can Form Intermolecular Hydrogen Bonds?.
Web this type of intermolecular bond is stronger than london dispersion forces with the same number of electrons. Hydrogen bonds have strengths ranging from 5 kj/mol to 50 kj/mol. Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? Web a hydrogen bond is an electrostatic attraction between a partially negative n or o atom and a partially positive hydrogen atom that is covalently bound to a different n or o atom.