Which Atom Is Most Likely To Form A Metallic Bond
Which Atom Is Most Likely To Form A Metallic Bond - Web which atom is most likely to form a metallic bond get the answers you need, now! The loss of metallic properties b. A metallic bond is a type of chemical bond similar to a covalent bond. Because metals are solid, their atoms are tightly packed in a regular. If atoms have similar electronegativities (the. However, it can also be observed between nonmetals and metals. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized. Web which atom is most likely to form a metallic bond? Its bonds are formed by large differences in electronegativity. Metallic bonds are the kind of bonds that forms between metallic atoms.
The loss of metallic properties b. Define electronegativity and assess the polarity of covalent bonds. Web which atom is most likely to form a metallic bond? Web a metallic bond is the way that metal atoms are kept together within a metal material. Web this bonding occurs primarily between nonmetals; A metallic bond is a type of chemical bond similar to a covalent bond. Ionic bonding results from the electrostatic attraction of oppositely. Web aluminum atom is most likely to form a metallic bond. The mercurous ion also exhibits metallic and covalent. Its bonds are formed by large differences in electronegativity.
Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized. Aluminum (al) helium (he) phosphorus (p) carbon (c) a lead is malleable, so it can be pounded into flat sheets. Web which atom is most likely to form a metallic bond? Metallic bonds are the kind of bonds that forms between metallic atoms. Web for example, covalently bonded gallium atoms tend to form crystal structures that are held together via metallic bonds. Define electronegativity and assess the polarity of covalent bonds. Which is most likely the result of millions of metal atoms crowding together so that molecular orbitals become combined? Ionic bonding results from the electrostatic attraction of oppositely. It has only metallic bonds between the atoms. If atoms have similar electronegativities (the.
Bonds Between Atoms Sciencetastic!
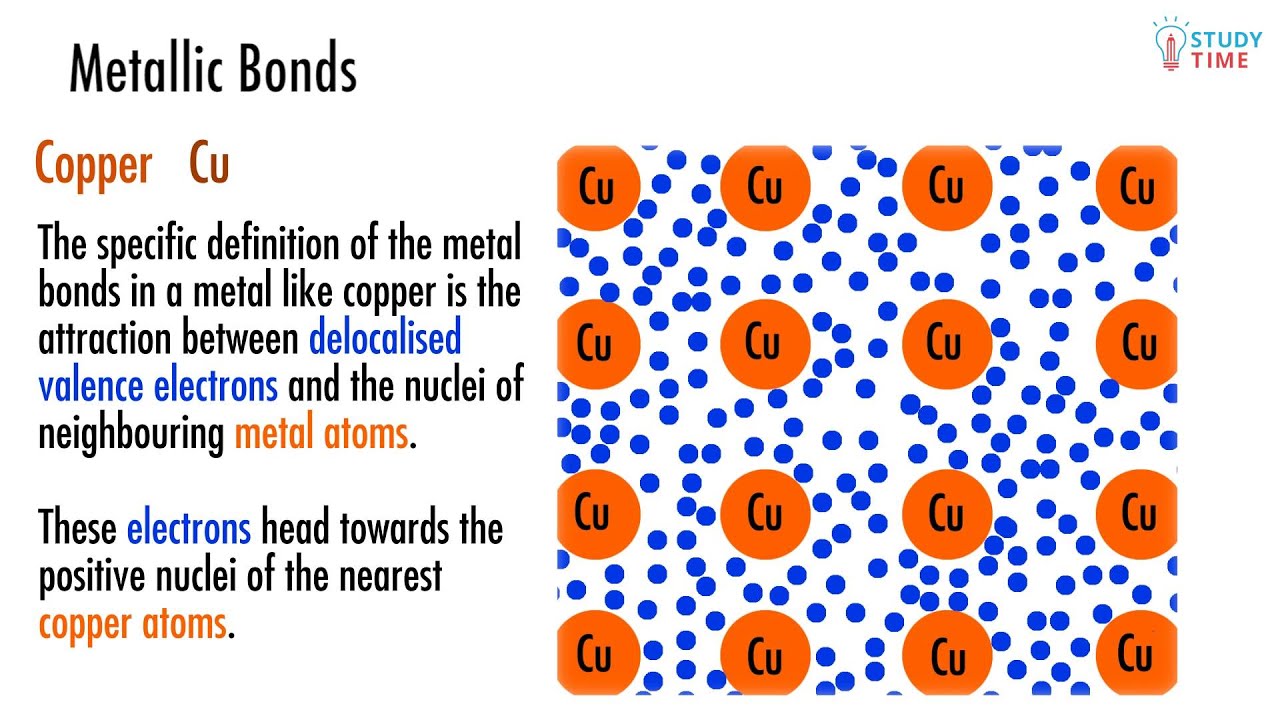
A kind of chemical bonding which generates between conduction electrons. Aluminium (al) atom is most likely to form a metallic bond. Web describe the formation of covalent bonds. Ionic bonding results from the electrostatic attraction of oppositely. Web this bonding occurs primarily between nonmetals;
1.6 Periodic Variations in Element Properties Chemistry for
A kind of chemical bonding which generates between conduction electrons. If atoms have similar electronegativities (the. Because metals are solid, their atoms are tightly packed in a regular. Metallic bonds are the kind of bonds that forms between metallic atoms. Web answer:aluminium (al) atom is most likely to form a metallic bond.explanation:a kind of chemical bonding which generates between conduction.
savvychemist Periodicity (2) Melting and boiling points of the
A kind of chemical bonding which generates between conduction electrons. Web which atom is most likely to form a metallic bond get the answers you need, now! Web aluminum atom is most likely to form a metallic bond. Web answer:aluminium (al) atom is most likely to form a metallic bond.explanation:a kind of chemical bonding which generates between conduction electrons and.
Why Do Most Atoms Form Chemical Bonds? Sciencing
Web aluminum atom is most likely to form a metallic bond. Web which atom is most likely to form a metallic bond? Web when one atom takes an electron away from another and the resulting positive and negative ions are attracted to each other, those atoms have formed an ionic bond. A kind of chemical bonding which generates between conduction.
Metallic Bonding Meaning, Properties, Factors Embibe
Define electronegativity and assess the polarity of covalent bonds. Web when one atom takes an electron away from another and the resulting positive and negative ions are attracted to each other, those atoms have formed an ionic bond. Web answer:aluminium (al) atom is most likely to form a metallic bond.explanation:a kind of chemical bonding which generates between conduction electrons and.
A carbon atom is most likely to form what kind of bond(s) with other
The mercurous ion also exhibits metallic and covalent. Web mostly, in the periodic table, left elements form metallic bonds, for example, zinc and copper. Web answer:aluminium (al) atom is most likely to form a metallic bond.explanation:a kind of chemical bonding which generates between conduction electrons and positively Aluminum (al) helium (he) phosphorus (p) carbon (c) a lead is malleable, so.
Metallic Substances (8/12) Atomic Structure NCEA Level 2 Chemistry
However, it can also be observed between nonmetals and metals. Web for example, covalently bonded gallium atoms tend to form crystal structures that are held together via metallic bonds. Ionic bonding results from the electrostatic attraction of oppositely. Web a metallic bond is the way that metal atoms are kept together within a metal material. Aluminum (al) helium (he) phosphorus.
Chemistry B.Sc Level How many types of chemical bond
Which is most likely the result of millions of metal atoms crowding together so that molecular orbitals become combined? Aluminium (al) atom is most likely to form a metallic bond. Web which atom is most likely to form a metallic bond get the answers you need, now! However, it can also be observed between nonmetals and metals. Web for example,.
What Is Metallic Bonding Metallic Bonding and Structure YouTube
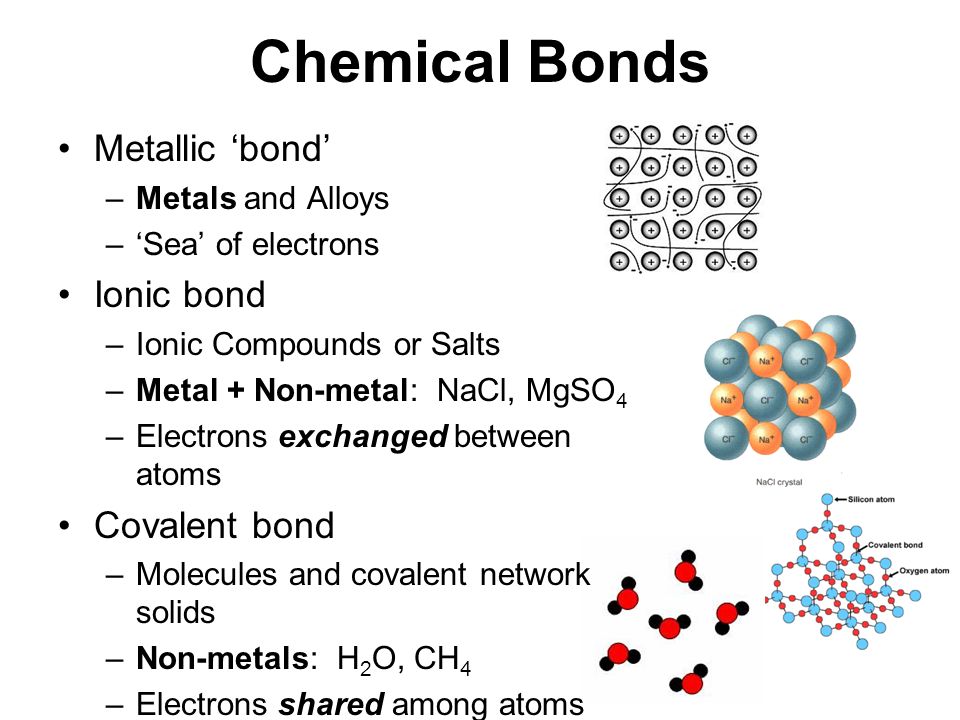
Its bonds are formed by large differences in electronegativity. Ionic bonding results from the electrostatic attraction of oppositely. Web which atom is most likely to form a metallic bond? Web a metallic bond is the way that metal atoms are kept together within a metal material. A metallic bond is a type of chemical bond similar to a covalent bond.
Metallic Bond — Formation & Compounds Expii
It has only covalent bonds between the atoms. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized. Web describe the formation of covalent bonds. Web which atom is most likely to form a metallic bond get the answers you need, now!.
Define Electronegativity And Assess The Polarity Of Covalent Bonds.
Web which atom is most likely to form a metallic bond? Which is most likely the result of millions of metal atoms crowding together so that molecular orbitals become combined? Web aluminum atom is most likely to form a metallic bond. The mercurous ion also exhibits metallic and covalent.
Web For Example, Covalently Bonded Gallium Atoms Tend To Form Crystal Structures That Are Held Together Via Metallic Bonds.
Because metals are solid, their atoms are tightly packed in a regular. The loss of metallic properties b. Web a metallic bond is the way that metal atoms are kept together within a metal material. It has only metallic bonds between the atoms.
Aluminum (Al) Helium (He) Phosphorus (P) Carbon (C) A Lead Is Malleable, So It Can Be Pounded Into Flat Sheets.
It has only covalent bonds between the atoms. Ionic bonding results from the electrostatic attraction of oppositely. Metallic bonds are the kind of bonds that forms between metallic atoms. Web answer:aluminium (al) atom is most likely to form a metallic bond.explanation:a kind of chemical bonding which generates between conduction electrons and positively
Aluminum (Al) Helium (He) Phosphorus (P) Carbon (C) Aluminum (Al) Is Most Likely To Form A Metallic Bond.
Web mostly, in the periodic table, left elements form metallic bonds, for example, zinc and copper. A kind of chemical bonding which generates between conduction electrons. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized. Web this bonding occurs primarily between nonmetals;






:max_bytes(150000):strip_icc()/artwork-of-a-graphene-sheet-685024423-5c49e1cdc9e77c0001b8e3e9.jpg)
