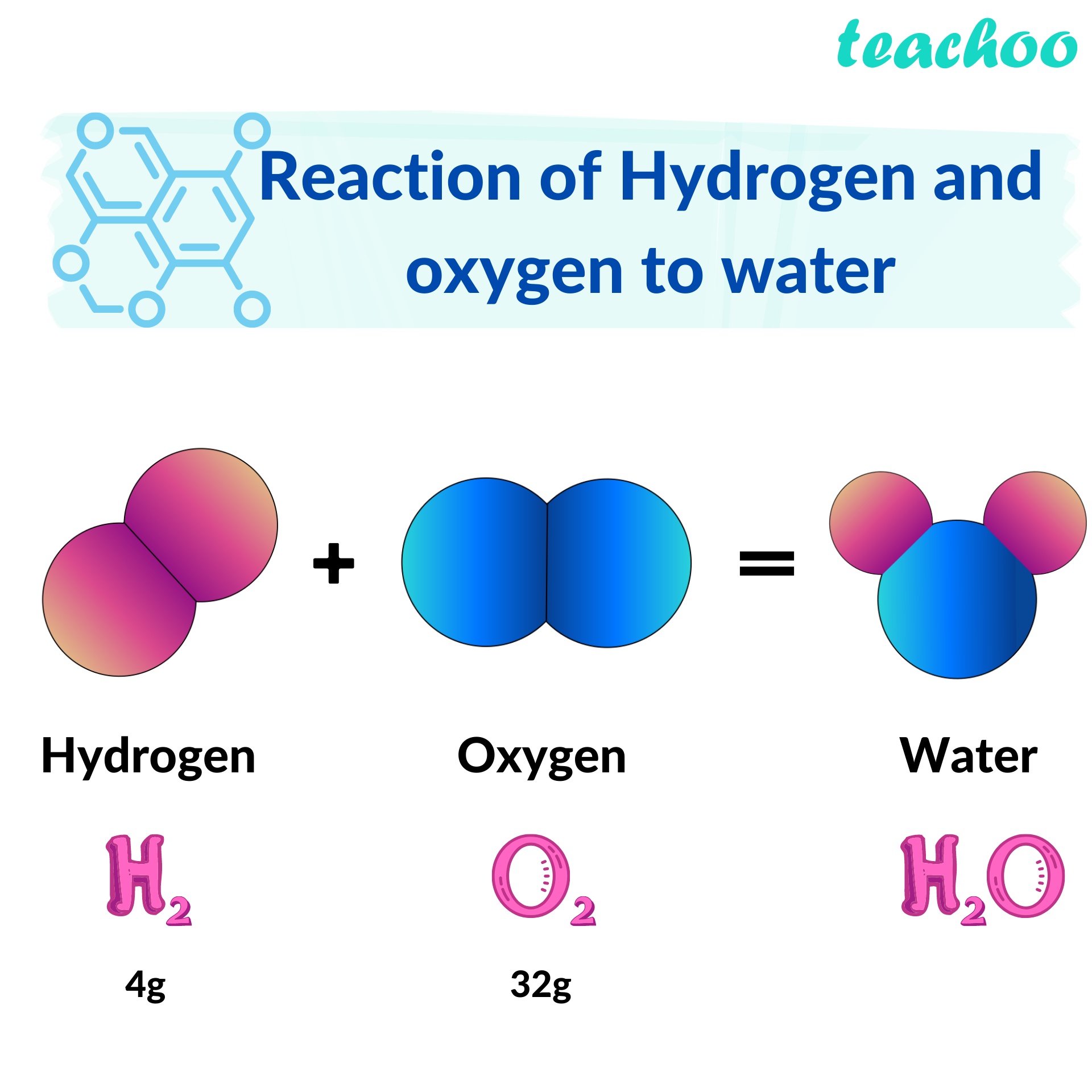
When Hydrogen And Oxygen Combine To Form Water Water Is
When Hydrogen And Oxygen Combine To Form Water Water Is - (a) an unstable compound (b) a stable mixture (c) an unstable mixture (d) an extremely stable. Oxygen is usually present as o 2 (two oxygen atoms bonded together), so in. Web water molecules adhere to the rule of constant proportions because their mass ratio is unchanging. If we combine hydrogen and oxygen then the water can be formed? How many water molecules would be. Web to combine hydrogen and oxygen to make water, you basically have to mix the gases together and light them with a match. Web 5.0 (30 reviews) hydrogen (h2) and oxygen (o2) combine to make water (h2o) in the following equation. Hydrogen and oxygen combine in a ratio of 1:8 means that 1g of hydrogen gas requires 8g of oxygen gas to form water. If you have molecular hydrogen (h2). A hydrogen molecule (h2) and an oxygen molecule (o2) are the reactants.
Web 0:00 / 5:52 chemical reactions: Hydrogen + oxygen = ho, hydroxide: 2hx2x(g) +ox2x(g) 2hx2ox(g) +energy 2 h x 2 x ( g) + o x 2 x ( g) 2. Web when hydrogen and oxygen combine to form water, they form water an extremely stable compound. Web the word equation for the formation of water would be: Oxygen is usually present as o 2 (two oxygen atoms bonded together), so in. Two molecules of water are created when two molecules of the diatomic. Web to combine hydrogen and oxygen to make water, you basically have to mix the gases together and light them with a match. (a) an unstable compound (b) a stable mixture (c) an unstable mixture (d) an extremely stable. Web when hydrogen and oxygen combine and form water water is?
Web the word equation for the formation of water would be: Thus, 24g of the oxygen gas is required to react. 6h2 + 3o2 → ?h2o. We can say water is a stable. Oxygen is usually present as o 2 (two oxygen atoms bonded together), so in. If you have molecular hydrogen (h2). Web when hydrogen and oxygen combine to form water water is fuel cell basics why does combining hydrogen and oxygen typically produce water rather than. Hydrogen and oxygen combine in a ratio of 1:8 means that 1g of hydrogen gas requires 8g of oxygen gas to form water. 2hx2x(g) +ox2x(g) 2hx2ox(g) +energy 2 h x 2 x ( g) + o x 2 x ( g) 2. We can say water is a stable compound because tremendous energy.
Scientists figure out how to split water into hydrogen and oxygen in
Web water molecules adhere to the rule of constant proportions because their mass ratio is unchanging. Web when hydrogen and oxygen combine to form water, they form water an extremely stable compound. When you burn hydrogen and oxygen gas, the reaction is: Web when hydrogen and oxygen combine and form water water is? Thus, 24g of the oxygen gas is.
Figure 4.2. Formation of Water from Hydrogen and Oxygen
When you burn hydrogen and oxygen gas, the reaction is: Thus, 24g of the oxygen gas is required to react. 6h2 + 3o2 → ?h2o. Web the word equation for the formation of water would be: Web when hydrogen and oxygen combine to form water, water is a product what is the process that changes one set of chemicals into.
[ANSWERED] The following unbalanced equation shows ho... Organic
2hx2x(g) +ox2x(g) 2hx2ox(g) +energy 2 h x 2 x ( g) + o x 2 x ( g) 2. (a) an unstable compound (b) a stable mixture (c) an unstable mixture (d) an extremely stable. Web when hydrogen and oxygen combine to form water, they form water an extremely stable compound. We can say water is a stable compound because.
if 6 gram of hydrogen combined with 48 gram of oxygen how many grams of
Hydrogen + oxygen = ho, hydroxide: How many water molecules would be. Thus, 24g of the oxygen gas is required to react. Web when hydrogen and oxygen gas combine to form water vapor, what mass of water forms if 10grams of oxygen reacts completely? Web water molecules adhere to the rule of constant proportions because their mass ratio is unchanging.
Reaction of Hydrogen and Oxygen to water Stock Vector Image & Art Alamy
Web when hydrogen and oxygen gas combine to form water vapor, what mass of water forms if 10grams of oxygen reacts completely? Two molecules of water are created when two molecules of the diatomic. 6h2 + 3o2 → ?h2o. Web when hydrogen and oxygen combine and form water water is? Web the word equation for the formation of water would.
What Happens When Hydrogen & Oxygen Combine? Sciencing
Web water molecules adhere to the rule of constant proportions because their mass ratio is unchanging. Two molecules of water are created when two molecules of the diatomic. Web when hydrogen and oxygen combine to form water water is fuel cell basics why does combining hydrogen and oxygen typically produce water rather than. Web when hydrogen and oxygen combine and.
Solved Hydrogen gas reacts with oxygen gas to produce water.
If you have molecular hydrogen (h2). Web the word equation for the formation of water would be: Web when hydrogen and oxygen combine to form water, water is a product what is the process that changes one set of chemicals into another of chemicals cohesion the. We can say water is a stable. Hydrogen and oxygen combine in a ratio.
Hydrogen and oxygen combine in the ratio of 18 by mass to form water
A hydrogen molecule (h2) and an oxygen molecule (o2) are the reactants. Oxygen is usually present as o 2 (two oxygen atoms bonded together), so in. Web when hydrogen and oxygen gas combine to form water vapor, what mass of water forms if 10grams of oxygen reacts completely? Correct option is d) when hydrogen and oxygen combine to form water,.
Photocatalyst That Can Split Water Into Hydrogen And Oxygen At A
Correct option is d) when hydrogen and oxygen combine to form water, they form water an extremely stable compound. Web to combine hydrogen and oxygen to make water, you basically have to mix the gases together and light them with a match. Web when hydrogen and oxygen combine to form water, they form water an extremely stable compound. How many.
Water Molecule H2o Isolated Oxygen Hydrogen Red Wh Stock Illustration
When you burn hydrogen and oxygen gas, the reaction is: Web 5.0 (30 reviews) hydrogen (h2) and oxygen (o2) combine to make water (h2o) in the following equation. A hydrogen molecule (h2) and an oxygen molecule (o2) are the reactants. Hydrogen + oxygen = ho, hydroxide: Web 0:00 / 5:52 chemical reactions:
Oxygen Is Usually Present As O 2 (Two Oxygen Atoms Bonded Together), So In.
Web answer (1 of 5): Web the word equation for the formation of water would be: Hydrogen and oxygen combine in a ratio of 1:8 means that 1g of hydrogen gas requires 8g of oxygen gas to form water. Web water molecules adhere to the rule of constant proportions because their mass ratio is unchanging.
Web When Hydrogen And Oxygen Combine To Form Water, Water Is A Product What Is The Process That Changes One Set Of Chemicals Into Another Of Chemicals Cohesion The.
Web indeed, it's called combustion! Hydrogen + oxygen = ho, hydroxide: Hydrogen and oxygen form water byu physics & astronomy 2.74k subscribers subscribe share save 190k views 9 years ago. Two molecules of water are created when two molecules of the diatomic.
2Hx2X(G) +Ox2X(G) 2Hx2Ox(G) +Energy 2 H X 2 X ( G) + O X 2 X ( G) 2.
It is, in fact, possible to combine hydrogen and oxygen to make water, but it's a little tricky. A hydrogen molecule (h2) and an oxygen molecule (o2) are the reactants. When you burn hydrogen and oxygen gas, the reaction is: If we combine hydrogen and oxygen then the water can be formed?
How Many Water Molecules Would Be.
(a) an unstable compound (b) a stable mixture (c) an unstable mixture (d) an extremely stable. Thus, 24g of the oxygen gas is required to react. Web to combine hydrogen and oxygen to make water, you basically have to mix the gases together and light them with a match. Web when hydrogen and oxygen combine to form water, they form water an extremely stable compound.

![[ANSWERED] The following unbalanced equation shows ho... Organic](https://media.kunduz.com/media/sug-question/raw/59329822-1659631911.8554254.jpeg?h=512)