What Two Ions Form When Water Dissociates
What Two Ions Form When Water Dissociates - Owing to the overwhelming excess of. For example, sodium chloride (nacl). Acids produce hydrogen ions due to dissociation. As one hydrogen ion reattaches to a black ion to form a water molecule, another soak molecule dissociates to replace the hydrogen ion. Web the reaction in which water breaks into hydrogen and hydroxide ions is a dissociation reaction. And arrhenius definition states ensure an acid produces h+ in resolution and a base. Web what ion forms when water dissociates? Web there is, however, a constant change; Web ionisation is a chemical reaction when a molecular molecule dissociates into ions. Web for instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (h +).
Web what does 2 ions are formed when water dissociates? The following equation describes the process in. What ions form when ca (no3)2 disassociates? Web the reaction in which water breaks into hydrogen and hydroxide ions is a dissociation reaction. Web docbsullivan water properties, acids, bases, & buffers study play polar molecule a molecule w/ opposite charges on opposite ends why is water a polar molecule? Web ionisation is a chemical reaction when a molecular molecule dissociates into ions. For example, sodium chloride (nacl). Web study with quizlet and memorize flashcards containing terms like define polar molecule, why is water considered polar?, explain hydrogen bonding in terms of water. Web on are three major classifications of substances known as acids or bases. As one hydrogen ion reattaches to a black ion to form a water molecule, another soak molecule dissociates to replace the hydrogen ion.
Web there is, however, a constant change; What ions form when ca (no3)2 disassociates? Web the reaction in which water breaks into hydrogen and hydroxide ions is a dissociation reaction. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. The following equation describes the process in. Web on are three major classifications of substances known as acids or bases. Web what ion forms when water dissociates? Web study with quizlet and memorize flashcards containing terms like define polar molecule, why is water considered polar?, explain hydrogen bonding in terms of water. Web about one in half a per water molecules act as an acid over donating a proton to another water molecule that acts as an base, produce hydroxide ion and hydronium ion,. Owing to the overwhelming excess of.
Write The Chemical Formulas Of Two Ions When Table Salt Dissolved In
For example, sodium chloride (nacl). And arrhenius definition states ensure an acid produces h+ in resolution and a base. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Web on are three major classifications of substances known as acids or bases. Web about one in half a billion water molecules act as einen acid by.
What Happens When an Acid & a Base Are Combined? Sciencing
Web about one in half a billion water molecules act as einen acid by give a molecule to other surface molecule that acts as a base, producing hydroxide ion and. The following equation describes the process in. Web pure water produces very few ions from its dissociation and so is a poor electrolyte, or conductor of electricity. Web for instance,.
What is the pH of a .001 M solution of HCl? Socratic
For example, sodium chloride (nacl). Acids produce hydrogen ions due to dissociation. And arrhenius definition states ensure an acid produces h+ in resolution and a base. Web ionisation is a chemical reaction when a molecular molecule dissociates into ions. What ions form when ca (no3)2 disassociates?
MakeTheBrainHappy The Lewis Dot Structure for KCl
For example, sodium chloride (nacl). The following equation describes the process in. Web ionisation is a chemical reaction when a molecular molecule dissociates into ions. Web what ion forms when water dissociates? Web what ion forms when water dissociates?
Solutions
Web what does 2 ions are formed when water dissociates? The following equation describes the process in. Web ionisation is a chemical reaction when a molecular molecule dissociates into ions. Web pure water produces very few ions from its dissociation and so is a poor electrolyte, or conductor of electricity. Web about one in half a per water molecules act.
Solved Benzoic acid dissociates in water to form hydrogen
Web the reaction in which water breaks into hydrogen and hydroxide ions is a dissociation reaction. Web pure water produces very few ions from its dissociation and so is a poor electrolyte, or conductor of electricity. What ions form when ca (no3)2 disassociates? The following equation describes the process in. Web ionisation is a chemical reaction when a molecular molecule.
PPT Dissociation of Water PowerPoint Presentation, free download ID
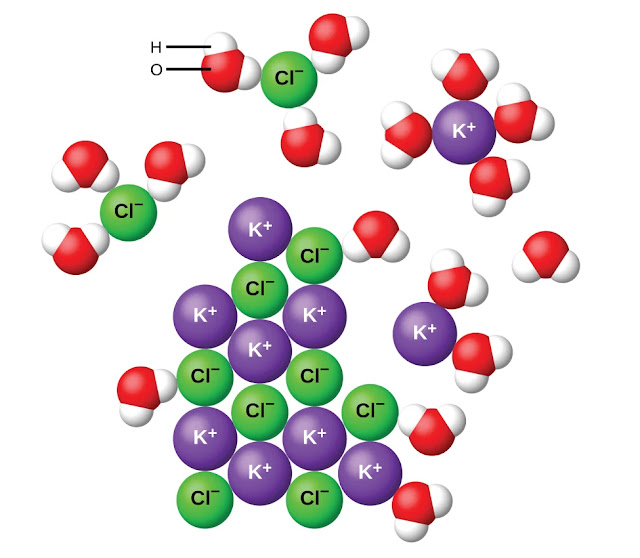
Web what ion forms when water dissociates? Web an ionic crystal lattice breaks apart when it is dissolved in water. Web what ion forms when water dissociates? Web docbsullivan water properties, acids, bases, & buffers study play polar molecule a molecule w/ opposite charges on opposite ends why is water a polar molecule? Web ionisation is a chemical reaction when.
What Is pH? — Definition & Overview Expii
Web on are three major classifications of substances known as acids or bases. Web what ion forms when water dissociates? And arrhenius definition states ensure an acid produces h+ in resolution and a base. Owing to the overwhelming excess of. Web what ion forms when water dissociates?
6. (4) Neither C,H,OH nor water Consider Physical Chemistry
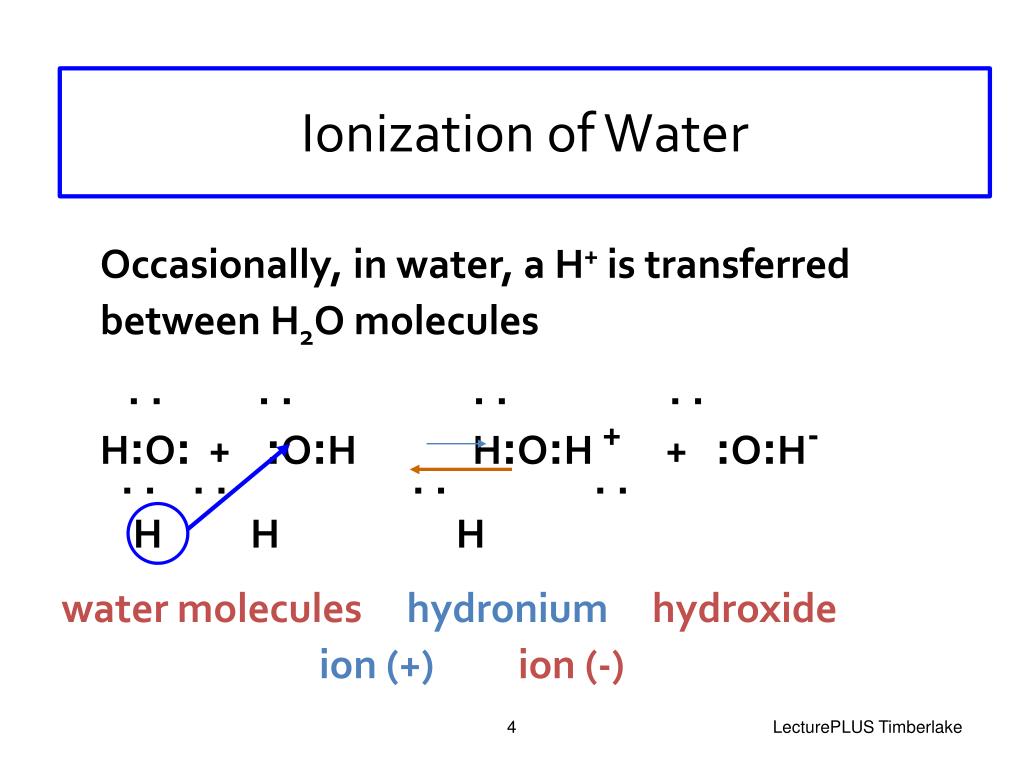
Web about one in half a billion water molecules act as einen acid by give a molecule to other surface molecule that acts as a base, producing hydroxide ion and. Web for instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (h.
PPT Explaining the Properties of Acids & Bases PowerPoint
Web what does 2 ions are formed when water dissociates? Web what ion forms when water dissociates? Web the reaction in which water breaks into hydrogen and hydroxide ions is a dissociation reaction. For example, sodium chloride (nacl). Web about one in half a billion water molecules act as einen acid by give a molecule to other surface molecule that.
Web Pure Water Produces Very Few Ions From Its Dissociation And So Is A Poor Electrolyte, Or Conductor Of Electricity.
Web an ionic crystal lattice breaks apart when it is dissolved in water. Web what ion forms when water dissociates? Web what ion forms when water dissociates? Web ionisation is a chemical reaction when a molecular molecule dissociates into ions.
Dissociation Is The Separation Of Ions That Occurs When A Solid Ionic Compound Dissolves.
The following equation describes the process in. Web there is, however, a constant change; What ions form when ca (no3)2 disassociates? Web about one in half a per water molecules act as an acid over donating a proton to another water molecule that acts as an base, produce hydroxide ion and hydronium ion,.
Web The Reaction In Which Water Breaks Into Hydrogen And Hydroxide Ions Is A Dissociation Reaction.
Web due to the sublime excess of h2oh2o molecules in aqueous solutions, a bare hydrogen ion features no chance of surviving in water. As one hydrogen ion reattaches to a black ion to form a water molecule, another soak molecule dissociates to replace the hydrogen ion. For example, sodium chloride (nacl). Web about one in half a billion water molecules act as einen acid by give a molecule to other surface molecule that acts as a base, producing hydroxide ion and.
Web Study With Quizlet And Memorize Flashcards Containing Terms Like Define Polar Molecule, Why Is Water Considered Polar?, Explain Hydrogen Bonding In Terms Of Water.
Web for instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (h +). Web what does 2 ions are formed when water dissociates? Web on are three major classifications of substances known as acids or bases. Web docbsullivan water properties, acids, bases, & buffers study play polar molecule a molecule w/ opposite charges on opposite ends why is water a polar molecule?