Two Point Form Of Arrhenius Equation
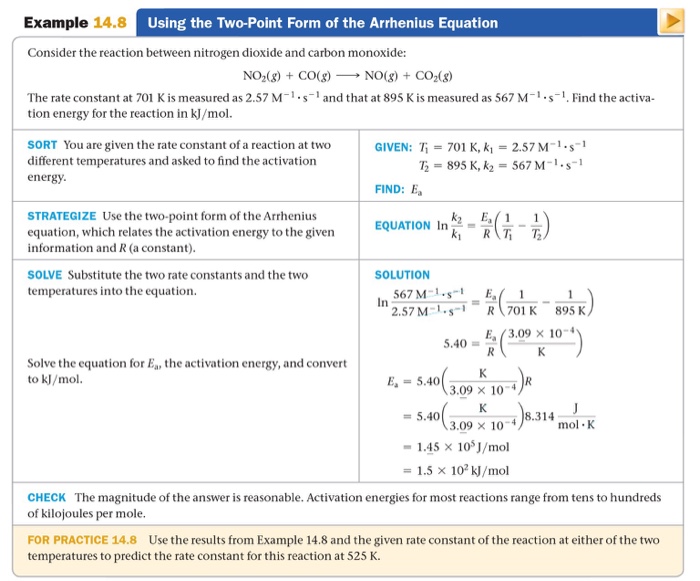
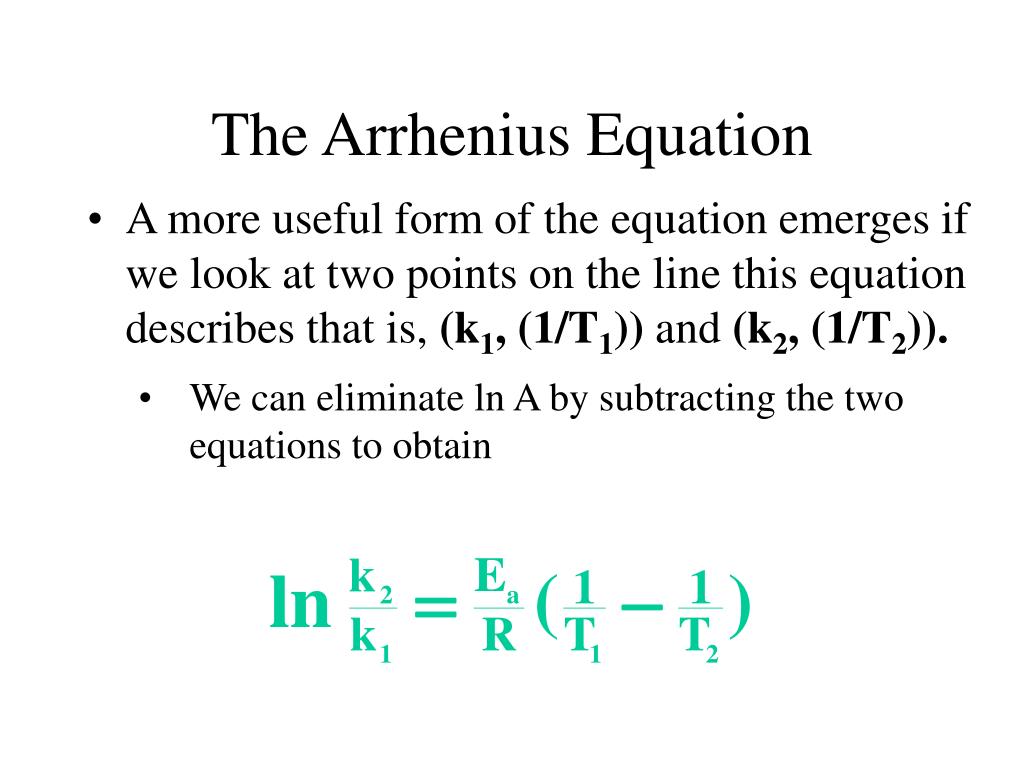
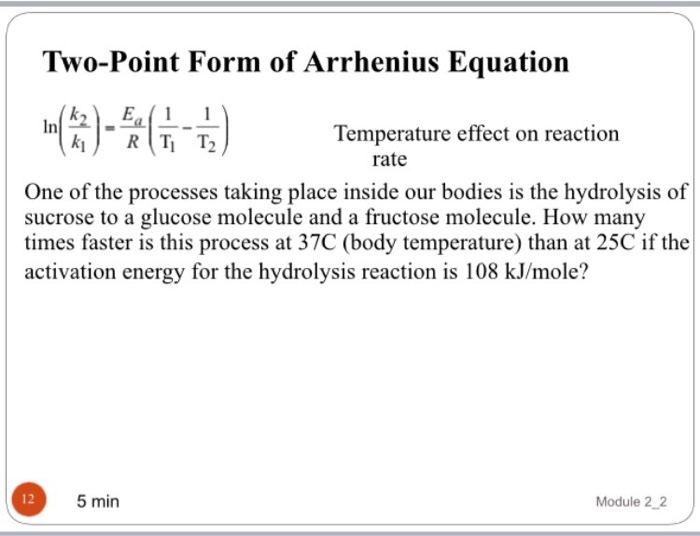
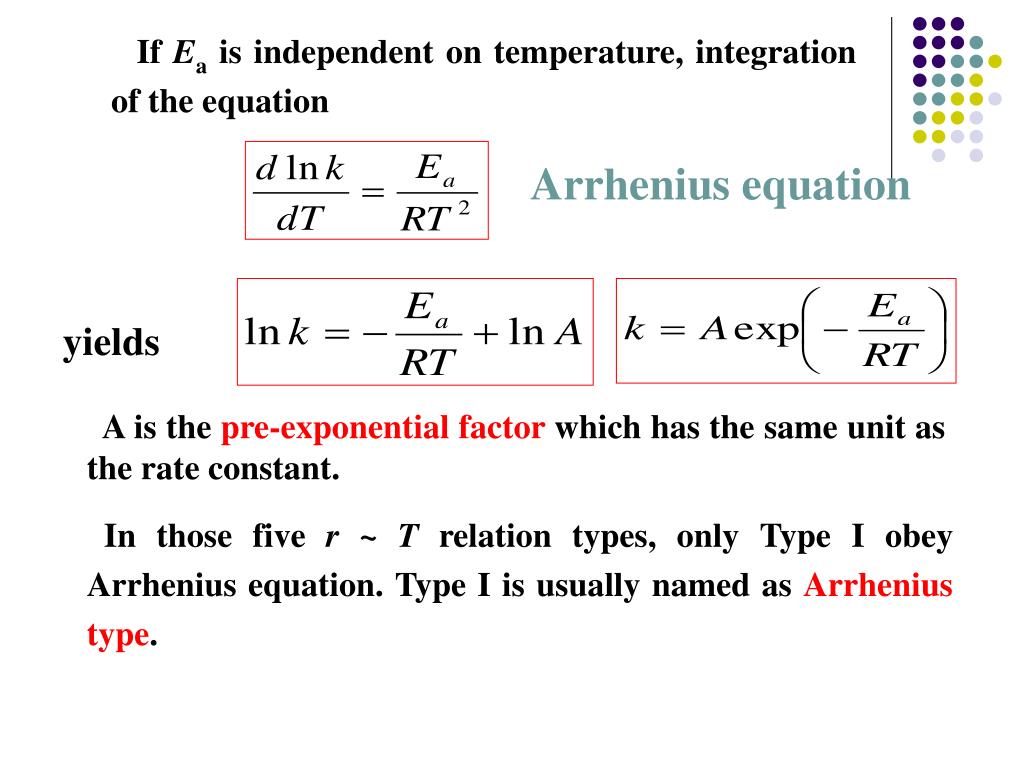
Two Point Form Of Arrhenius Equation - At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: (6.2.3.4.2) ln k = − e a r t + ln a Lnk = ln(ae − ea / rt) = lna + ln(e − ea / rt) = (− ea r)(1 t) + lna. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. Web the arrhenius equation, k = ae − ea / rt. For a second order reaction (of the form: Web two point arrhenius equation. Ea show steps k1 show steps k2 show steps t1 show steps t2. Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. Subtracting equation (i) from the equation (ii), we get.
If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: Web the arrhenius equation, k = ae − ea / rt. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. Web two point arrhenius equation. Web k = a e − e a r t arrhenius equation per molecule k = a e − e a k t linearized arrhenius equation the arrhenius equation (equation 6.2.3.4.1) can be rearranged to deal with specific situations. Amanda balcerzak's chemistry headquarters 136 subscribers this video shows how to. Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: (6.2.3.4.2) ln k = − e a r t + ln a Subtracting equation (i) from the equation (ii), we get.
Web the arrhenius equation, k = ae − ea / rt. Ea show steps k1 show steps k2 show steps t1 show steps t2. Web k = a e − e a r t arrhenius equation per molecule k = a e − e a k t linearized arrhenius equation the arrhenius equation (equation 6.2.3.4.1) can be rearranged to deal with specific situations. (6.2.3.4.2) ln k = − e a r t + ln a Subtracting equation (i) from the equation (ii), we get. Amanda balcerzak's chemistry headquarters 136 subscribers this video shows how to. At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: Lnk = ln(ae − ea / rt) = lna + ln(e − ea / rt) = (− ea r)(1 t) + lna. Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant.
I Need Help With For Practice 14.8. I'm Not Sure H...
Lnk = ln(ae − ea / rt) = lna + ln(e − ea / rt) = (− ea r)(1 t) + lna. Web the arrhenius equation, k = ae − ea / rt. If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: For a second order reaction (of the.
TwoPoint Form for Arrhenius Equation YouTube
Web the arrhenius equation, k = ae − ea / rt. Web k = a e − e a r t arrhenius equation per molecule k = a e − e a k t linearized arrhenius equation the arrhenius equation (equation 6.2.3.4.1) can be rearranged to deal with specific situations. Web two point arrhenius equation. Web the arrhenius activation energy.
PPT Halflife PowerPoint Presentation, free download ID1826480
Amanda balcerzak's chemistry headquarters 136 subscribers this video shows how to. Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. Web k = a e − e a r t arrhenius equation per molecule k = a e − e a k t linearized arrhenius equation.
Chapter13 chemical
Ea show steps k1 show steps k2 show steps t1 show steps t2. Web two point arrhenius equation. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. At two different temperatures t 1 and t 2, the corresponding values of rate constants k.
arrhenius equation Yahoo Image Search Results Gas constant
Web the arrhenius equation, k = ae − ea / rt. For a second order reaction (of the form: Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. Subtracting equation (i) from the equation (ii), we get. At two different temperatures t 1.
13.4 The Arrhenius Equation YouTube
Amanda balcerzak's chemistry headquarters 136 subscribers this video shows how to. Subtracting equation (i) from the equation (ii), we get. Web the arrhenius equation, k = ae − ea / rt. If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: Web the arrhenius equation is a formula that describes.
Two point arrhenius equation, Easy derivation, 3 application
Subtracting equation (i) from the equation (ii), we get. Web k = a e − e a r t arrhenius equation per molecule k = a e − e a k t linearized arrhenius equation the arrhenius equation (equation 6.2.3.4.1) can be rearranged to deal with specific situations. Ea show steps k1 show steps k2 show steps t1 show steps.
Solved TwoPoint Form of Arrhenius Equation In N2.
Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: (6.2.3.4.2) ln k = − e a r t.
Chapter13 chemical
If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: Amanda balcerzak's chemistry headquarters 136 subscribers this video shows how to. Web the arrhenius equation, k = ae − ea / rt. Subtracting equation (i) from the equation (ii), we get. For a second order reaction (of the form:
PPT temperaturedependence of reaction rate Arrhenius equation
Subtracting equation (i) from the equation (ii), we get. For a second order reaction (of the form: Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. (6.2.3.4.2) ln k = − e a r t + ln a At two different temperatures t 1 and t.
Subtracting Equation (I) From The Equation (Ii), We Get.
Ea show steps k1 show steps k2 show steps t1 show steps t2. If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: Web two point arrhenius equation. Lnk = ln(ae − ea / rt) = lna + ln(e − ea / rt) = (− ea r)(1 t) + lna.
Web K = A E − E A R T Arrhenius Equation Per Molecule K = A E − E A K T Linearized Arrhenius Equation The Arrhenius Equation (Equation 6.2.3.4.1) Can Be Rearranged To Deal With Specific Situations.
Web the arrhenius equation, k = ae − ea / rt. At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: (6.2.3.4.2) ln k = − e a r t + ln a For a second order reaction (of the form:
Web The Arrhenius Activation Energy For Two Temperature Calculator Uses The Arrhenius Equation To Compute Activation Energy Based On Two Temperatures And Two Reaction Rate Constants.
Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. Amanda balcerzak's chemistry headquarters 136 subscribers this video shows how to.