The Least Penetrating Form Of Radiation Is
The Least Penetrating Form Of Radiation Is - They differ in mass, energy and how. Alpha particles present a health threat only when they are taken internally through. Web gamma rays are the most energetic but least penetrating form of ionizing radiation. Exposure to cosmic rays has no adverse human health impact. The most penetrating form of. The atomic numbers and mass numbers in a nuclear equation must be balanced. They can only be stopped by thick layers of concrete or. The least penetrating form of radiation is alpha as it only travels 10cm in air and is stopped by paper. Which is the least penetrating? Alpha is also the most ionising, beta is less.
Alpha particles beta particles (electrons) least. The most penetrating form of. Exposure to cosmic rays has no adverse human health impact. Web gamma rays are the most energetic but least penetrating form of ionizing radiation. The least penetrating form of radiation is _______. Alpha, beta, neutrons, and electromagnetic waves such as gamma rays. Web a nuclear reaction is one that changes the structure of the nucleus of an atom. Web what is the change in atomic mass when an atom emits gamma radiation? Which is the least penetrating? Web gamma rays are the most penetrating form of radiation because they have the highest energy and smallest wavelength.
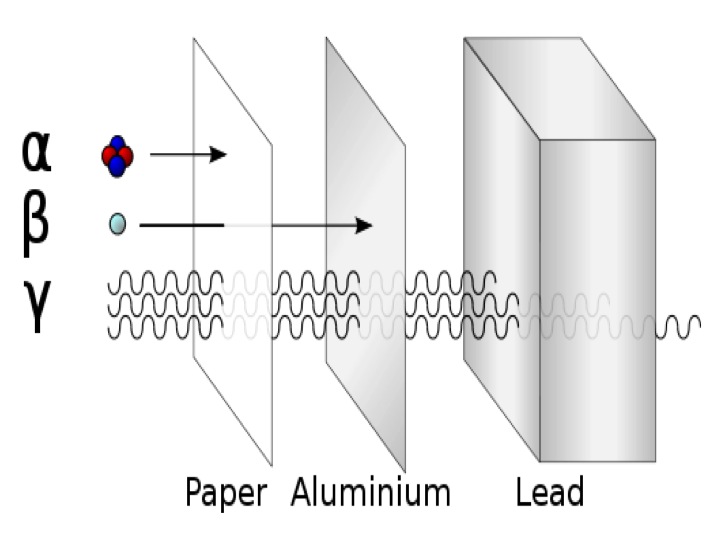
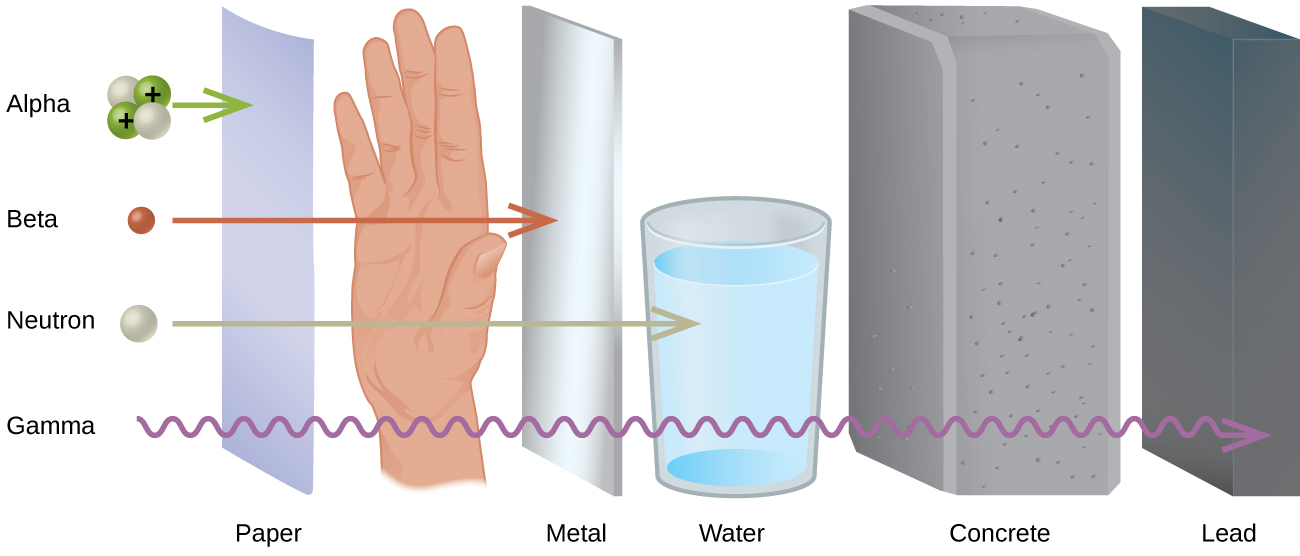
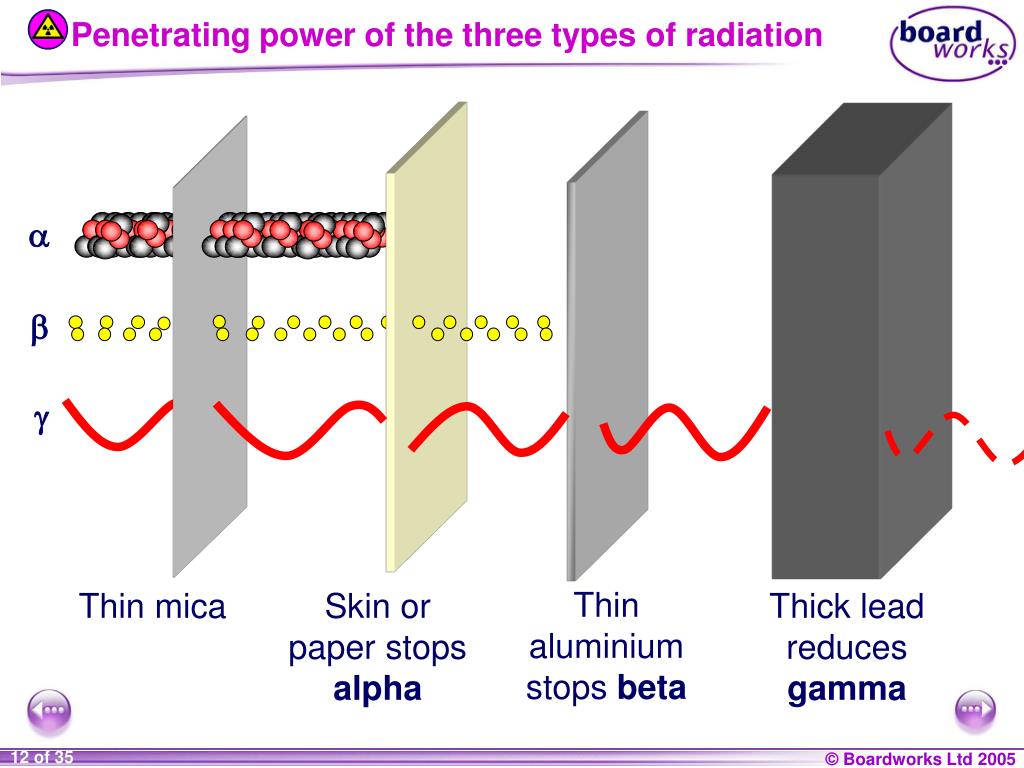
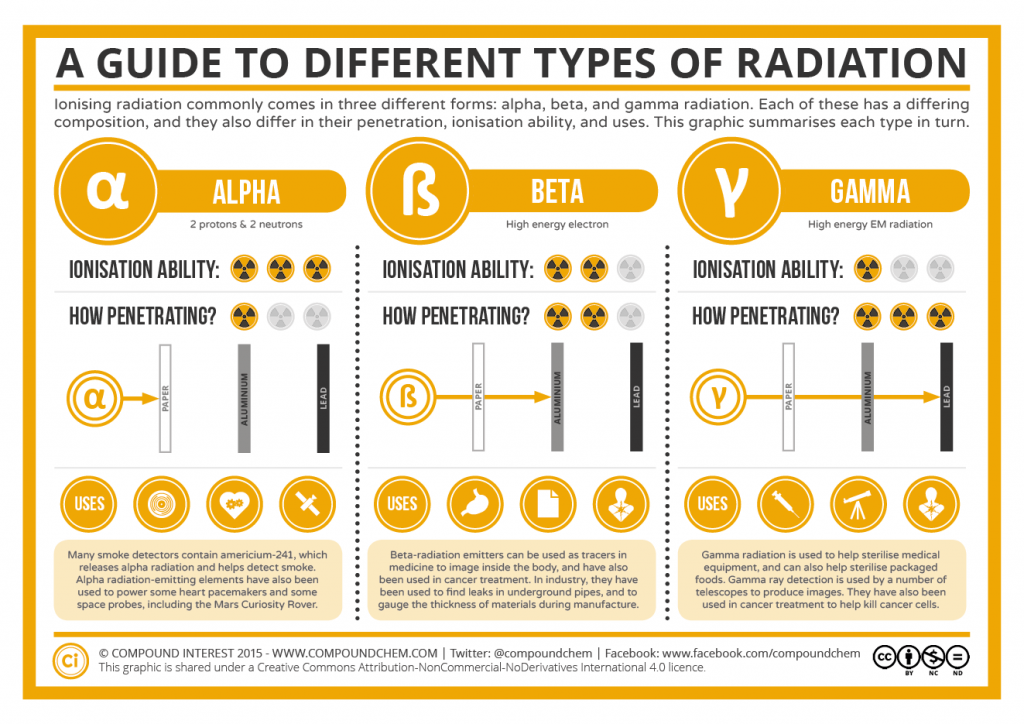
Web polaris emits peak radiation at 485 nm wavelength. Which is the least penetrating? They are part of the electromagnetic. Gamma rays are caused by changes within the nucleus. 0 what particle is emitted in. They can only be stopped by thick layers of concrete or. Web the illustration shows the penetrating power of different types of ionizing radiation, ranging from the least penetrating alpha particles to the most penetrating neutrons. Web gamma rays are the most penetrating form of radiation because they have the highest energy and smallest wavelength. Web what is the change in atomic mass when an atom emits gamma radiation? The types of radiation which are alpha, beta and gamma radiation have different penetration.
2.2 Electrons and other Discoveries Chemwiki
They are part of the electromagnetic. The most penetrating form of. Web which of the following forms of radiation is the least penetrating? Y which of the following statements is true. Α which of the following forms of radiation is the most penetrating?
Blue Collar Prepping Ionizing Radiation for Dummies
Web answered the least penetrating form of radiation consists of___. The atomic numbers and mass numbers in a nuclear equation must be balanced. The least penetrating form of radiation is _______. Gamma rays are the most energetic but least penetrating form of ionizing radiation. Beta radiation from radioactive decay can be stopped with a few centimeters of plastic or a.
Power Of Various Types Of Radiation Stock Illustration
Web what is the change in atomic mass when an atom emits gamma radiation? They can only be stopped by thick layers of concrete or. The most penetrating form of. To add, polaris, commonly the north star or pole star, is the brightest star in the constellation of ursa minor. Y which of the following statements is true.
Radon and Nuclear Chemistry Chemistry LibreTexts
Web learn test match created by kirstenf7 terms in this set (5) an unstable nucleus ___. Web a nuclear reaction is one that changes the structure of the nucleus of an atom. Web the illustration shows the penetrating power of different types of ionizing radiation, ranging from the least penetrating alpha particles to the most penetrating neutrons. To add, polaris,.
What Type of Radiation Is the Most Sciencing
Alpha particles beta particles (electrons) least. 0 what particle is emitted in. Alpha, beta, neutrons, and electromagnetic waves such as gamma rays. Web it is more penetrating than alpha radiation but less than gamma. The least penetrating form of radiation is alpha as it only travels 10cm in air and is stopped by paper.
What is radiation? & Particulate radiations [with
Web polaris emits peak radiation at 485 nm wavelength. Which is the least penetrating? They are part of the electromagnetic. The most penetrating form of. Alpha, beta, neutrons, and electromagnetic waves such as gamma rays.
21.6 Los efectos biológicos de la radiación LibreTexts Español
Web answered the least penetrating form of radiation consists of___. To add, polaris, commonly the north star or pole star, is the brightest star in the constellation of ursa minor. Web gamma rays are the most energetic but least penetrating form of ionizing radiation. They can only be stopped by thick layers of concrete or. They are part of the.
Alpha Beta And Gamma Radiation Power All About Radiation
Web the illustration shows the penetrating power of different types of ionizing radiation, ranging from the least penetrating alpha particles to the most penetrating neutrons. The types of radiation which are alpha, beta and gamma radiation have different penetration. Beta radiation from radioactive decay can be stopped with a few centimeters of plastic or a few millimeters of. Web which.
Solved Be 4. Calculate the mass of the missing isotope
0 what particle is emitted in. Web polaris emits peak radiation at 485 nm wavelength. Web the illustration shows the penetrating power of different types of ionizing radiation, ranging from the least penetrating alpha particles to the most penetrating neutrons. The correct answer is option (c) alpha radiation explanation: Y which of the following statements is true.
Recursos física nuclear FiQuiPedia
Exposure to cosmic rays has no adverse human health impact. Web which of the following forms of radiation is the least penetrating? Which is the least penetrating? Emits energy when it decays the charge on a gamma ray is ___. The least penetrating form of radiation is _______.
Y Which Of The Following Statements Is True.
Web the illustration shows the penetrating power of different types of ionizing radiation, ranging from the least penetrating alpha particles to the most penetrating neutrons. Beta radiation from radioactive decay can be stopped with a few centimeters of plastic or a few millimeters of. Web gamma rays are the most penetrating form of radiation because they have the highest energy and smallest wavelength. To add, polaris, commonly the north star or pole star, is the brightest star in the constellation of ursa minor.
Gamma Rays Are Caused By Changes Within The Nucleus.
Web what is the change in atomic mass when an atom emits gamma radiation? Emits energy when it decays the charge on a gamma ray is ___. Alpha particles beta particles (electrons) least. Web which one of the following forms of radiation is most penetrating?
The Most Penetrating Form Of.
Which is the least penetrating? The least penetrating form of radiation is alpha as it only travels 10cm in air and is stopped by paper. Web which of the following forms of radiation is the least penetrating? The least penetrating form of radiation is _______.
They Are Part Of The Electromagnetic.
They differ in mass, energy and how. Web answered the least penetrating form of radiation consists of___. Alpha particles present a health threat only when they are taken internally through. The atomic numbers and mass numbers in a nuclear equation must be balanced.