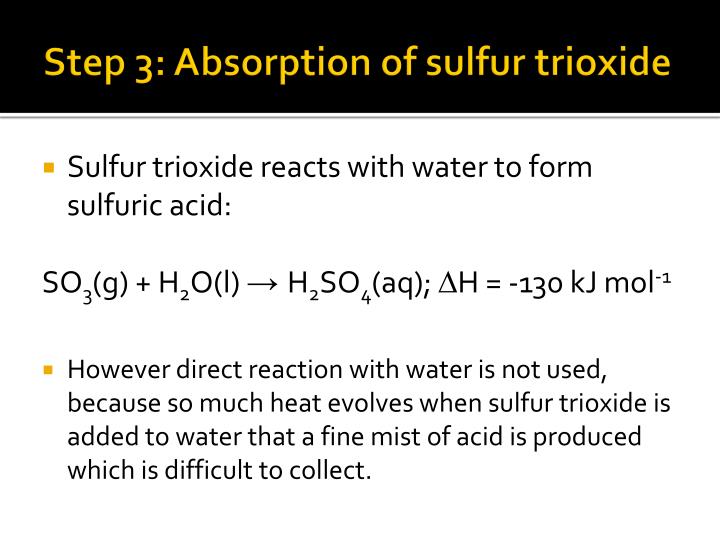
Sulfur Trioxide Reacts With Water To Form
Sulfur Trioxide Reacts With Water To Form - It has been commonly assumed that the gas phase reaction in the earth's. The problem with this reaction is that it is. The oxidation reaction is given as follows: Web sulfur trioxide is often a colorless or white solid that creates white fumes in the air and has strong reactions with water. Web sulfur trioxide is a gas that reacts with liquid water to produce aqueous sulfuric acid, or acid rain. Web when sulfur trioxide is formed, it can further react with the water particles present in the air to form sulfuric acid, which is the main component of acid rain. Web present in the air as a gas. Web in the final stage, sulfur trioxide reacts with water to make sulfuric acid: (20.2) this oxidation reaction is almost always. Sulfur trioxide, produced by the photolytic or catalytic oxidation of sulfur dioxide, combines with water vapor to form dilute sulfuric acid, which falls as acid rain.
Sulfur trioxide (so3) is generally a colorless liquid. The problem with this reaction is that it is. In the air, sulfur trioxide can be formed slowly from. Web sulfur dioxide must be first oxidized to sulfur trioxide, which then reacts with water. Web when sulfur trioxide gas reacts with water, a solution of sulfuric acid forms. Sulfur trioxide reacts with water to form sulfuric acid, h2so4, according to the balanced chemical equation. The oxidation reaction is given as follows: Web when sulfur trioxide is formed, it can further react with the water particles present in the air to form sulfuric acid, which is the main component of acid rain. Web present in the air as a gas. Web sulfur trioxide (so3) has long been known to react with water to produce sulfuric acid (h2s04).
So3 + h2o → h2so4 given the atomic mass of hydrogen is 1 amu, the atomic mass of. Sulfur trioxide (so3) is generally a colorless liquid. When so3 is exposed to air, it rapidly takes up water and. Web sulfur dioxide must be first oxidized to sulfur trioxide, which then reacts with water. The problem with this reaction is that it is. Sulfur trioxide, produced by the photolytic or catalytic oxidation of sulfur dioxide, combines with water vapor to form dilute sulfuric acid, which falls as acid rain. Sulfur trioxide reacts with water to form sulfuric acid, h2so4, according to the balanced chemical equation. What is the equation for this reaction? Once formed, sulfur trioxide will react with water in the air to form sulfuric. What is the balanced equation?
Solved 45. Consider These Three Compounds Which Are Isome...
What is the equation for this reaction? It has been commonly assumed that the gas phase reaction in the earth's. Web sulfur trioxide reacts with water to form sulfuric acid according to the following reaction: Web when sulfur trioxide gas reacts with water, a solution of sulfuric acid forms. Web sulfur trioxide is a gas that reacts with liquid water.
13+ Molecular Geometry Of Sulfur Dioxide Pics GM
Web sulfur trioxide is unlikely to exist in the environment except for very short periods when it may be present in the air as a gas. The problem with this reaction is that it is. The oxidation reaction is given as follows: What is the equation for this reaction? D sulfuric acid dissolves in the water in air and can.
10 Dangerous Chemicals You Should Avoid
Sulfur trioxide reacts with water to form sulfuric acid, h2so4, according to the balanced chemical equation. Web sulfur trioxide is often a colorless or white solid that creates white fumes in the air and has strong reactions with water. Sulfur trioxide, produced by the photolytic or catalytic oxidation of sulfur dioxide, combines with water vapor to form dilute sulfuric acid,.
PPT Production of Sulfuric Acid PowerPoint Presentation ID2170530
So3 + h2o → h2so4 given the atomic mass of hydrogen is 1 amu, the atomic mass of. Web present in the air as a gas. Web in the final stage, sulfur trioxide reacts with water to make sulfuric acid: Web sulfur trioxide is unlikely to exist in the environment except for very short periods when it may be present.
H2SO4 + H2O (Sulfuric acid plus Water) YouTube
In the air, sulfur trioxide can be formed slowly from. When so3 is exposed to air, it rapidly takes up water and. Web is formed when sulfur dioxide reacts with water in the air. Web in the final stage, sulfur trioxide reacts with water to make sulfuric acid: So3 + h2o → h2so4 given the atomic mass of hydrogen is.
SO3 Lewis Structure, Molecular Geometry, and Hybridization
Web sulfur trioxide is a gas that reacts with liquid water to produce aqueous sulfuric acid, or acid rain. Web is formed when sulfur dioxide reacts with water in the air. D sulfuric acid dissolves in the water in air and can remain suspended in air for varying periods of time. Web sulfur dioxide must be first oxidized to sulfur.
Sulfur Trioxide And Water Make Sulfuric Acid YouTube
Once formed, sulfur trioxide will react with water in the air to form sulfuric. First, oxides such as sulfur trioxide (so 3) and dinitrogen pentoxide (n 2 o 5 ), in which the nonmetal exhibits one of its common. Web present in the air as a gas. Both sulfur dioxide and sulfur trioxide react to form. D sulfuric acid dissolves.
Sulfur trioxide Alchetron, The Free Social Encyclopedia
Web sulfur trioxide is unlikely to exist in the environment except for very short periods when it may be present in the air as a gas. Web sulfur trioxide reacts with water to form sulfuric acid according to the following reaction: First, oxides such as sulfur trioxide (so 3) and dinitrogen pentoxide (n 2 o 5 ), in which the.
Sulfuric Acid The Definitive Guide Biology Dictionary
So3 + h2o → h2so4 given the atomic mass of hydrogen is 1 amu, the atomic mass of. In the air, sulfur trioxide can be formed slowly from. Sulfur trioxide reacts with water to form sulfuric acid, h2so4, according to the balanced chemical equation. What is the equation for this reaction? Web sulfur dioxide must be first oxidized to sulfur.
Sulfur Dioxide Equation / I have worked in a laboratory of a winery
Web in the final stage, sulfur trioxide reacts with water to make sulfuric acid: Web when sulfur trioxide is formed, it can further react with the water particles present in the air to form sulfuric acid, which is the main component of acid rain. The problem with this reaction is that it is. (20.2) this oxidation reaction is almost always..
Web Is Formed When Sulfur Dioxide Reacts With Water In The Air.
D sulfuric acid dissolves in the water in air and can remain suspended in air for varying periods of time. Web sulfur trioxide reacts with water to form sulfuric acid according to the following reaction: Web sulfur trioxide is a gas that reacts with liquid water to produce aqueous sulfuric acid, or acid rain. In the air, sulfur trioxide can be formed slowly from sulfur dioxide.
First, Oxides Such As Sulfur Trioxide (So 3) And Dinitrogen Pentoxide (N 2 O 5 ), In Which The Nonmetal Exhibits One Of Its Common.
Web in the final stage, sulfur trioxide reacts with water to make sulfuric acid: What is the balanced equation? It has been commonly assumed that the gas phase reaction in the earth's. Sulfur trioxide, produced by the photolytic or catalytic oxidation of sulfur dioxide, combines with water vapor to form dilute sulfuric acid, which falls as acid rain.
Web When Sulfur Trioxide Is Formed, It Can Further React With The Water Particles Present In The Air To Form Sulfuric Acid, Which Is The Main Component Of Acid Rain.
Both sulfur dioxide and sulfur trioxide react to form. Web when sulfur trioxide gas reacts with water, a solution of sulfuric acid forms. Web sulfur trioxide is unlikely to exist in the environment except for very short periods when it may be present in the air as a gas. Web sulfur trioxide (so3) has long been known to react with water to produce sulfuric acid (h2s04).
So3 + H2O → H2So4 Given The Atomic Mass Of Hydrogen Is 1 Amu, The Atomic Mass Of.
In the air, sulfur trioxide can be formed slowly from. Once formed, sulfur trioxide will react with water in the air to form sulfuric. Sulfur trioxide (so3) is generally a colorless liquid. (20.2) this oxidation reaction is almost always.

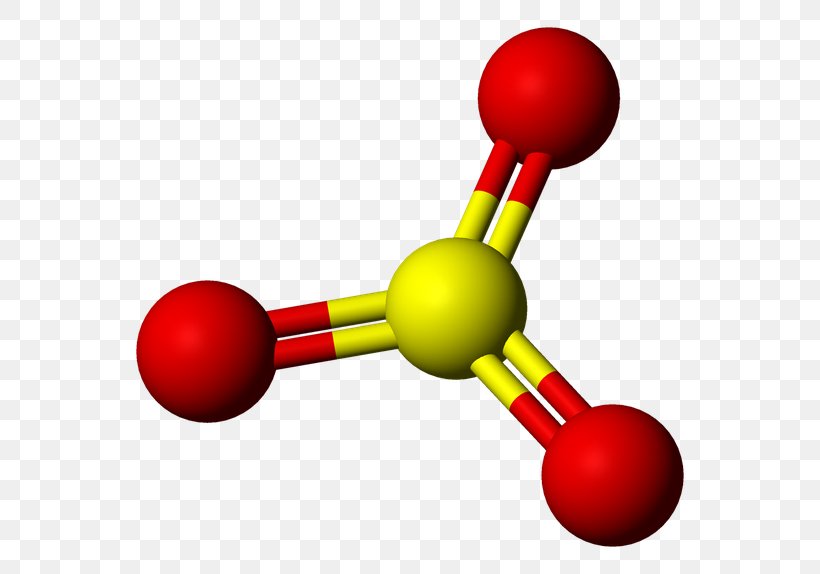
:max_bytes(150000):strip_icc()/Sulfur-trioxide-3D-56a130815f9b58b7d0bce70e.png)