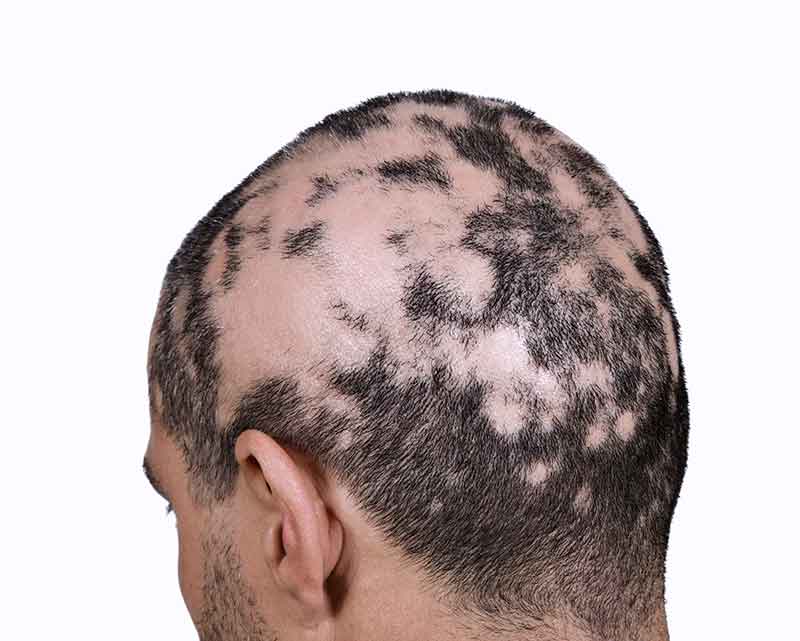
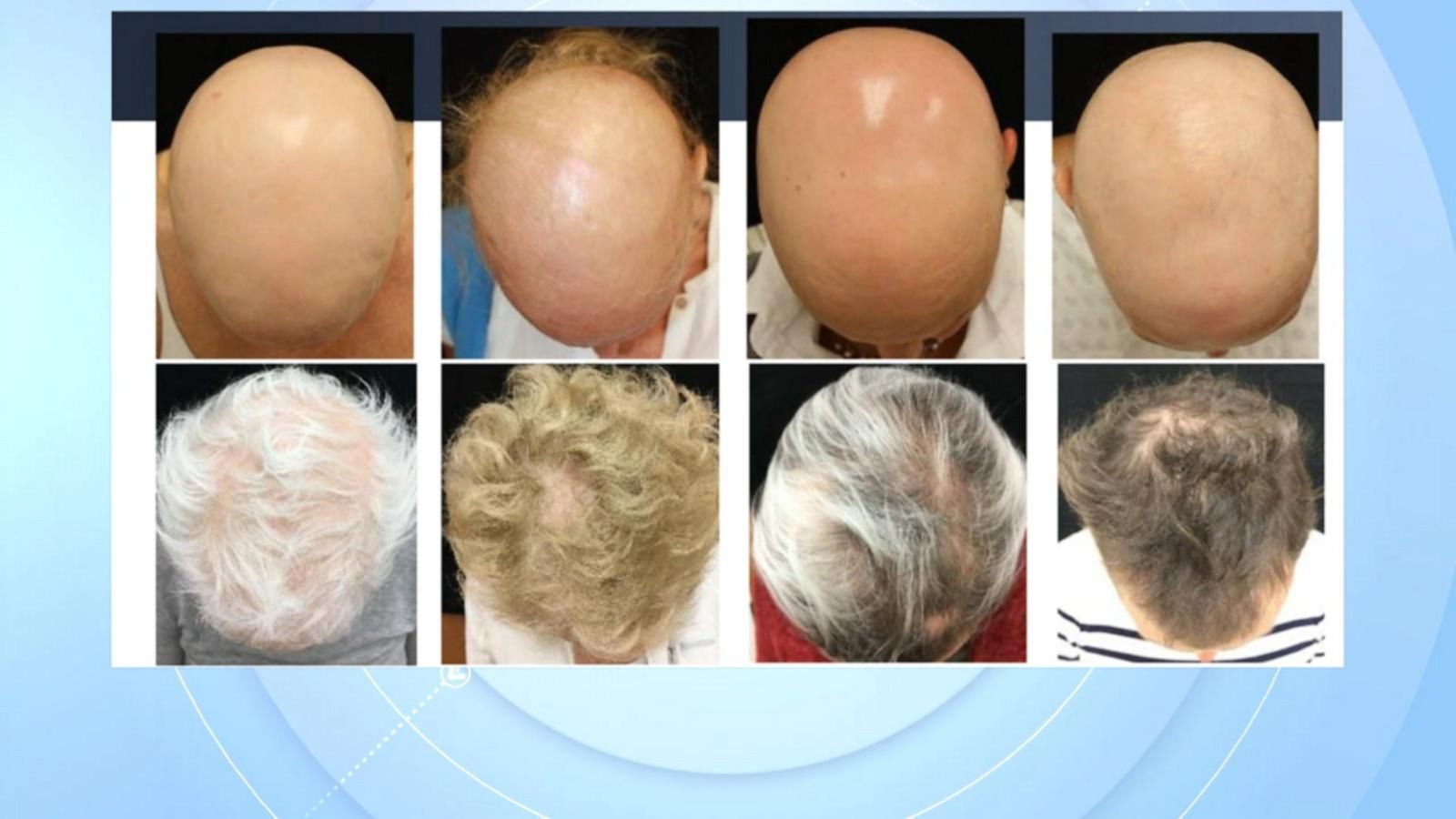
Olumiant Enrollment Form Alopecia
Olumiant Enrollment Form Alopecia - Food and drug administration (fda). Ad view efficacy, safety, full prescribing information, and boxed warning. Download support resources, including a doctor discussion guide. Web june 15, 2022 allure/soleil summer on monday, june 13, the u.s. Ad view prescribing info, safety info & boxed warning. Web olumiant (baricitinib) is a new treatment for hair loss due to severe alopecia areata, a condition in which the immune system attacks hair follicles. Today, history was made as the u.s. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant. Web alopecia areata causes hair loss that can happen unpredictably. But some current treatments (though not.
Today, history was made as the u.s. Food and drug administration (fda) for adults with severe alopecia areata (aa) (1). Web fda approves olumiant™ (baricitinib) for adults with severe alopecia areata. Hair loss can affect your scalp or any place where hair grows on your body. Web impairment (estimated glomerular filtration rate (gfr) between 30 and olumiant</strong> is not recommended. Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an. Ad schedule a free consultation with one of our hair restoration experts today! Ad view prescribing info, safety info & boxed warning. Web june 13, 2022 español today, the u.s. Not recommended for use in combination with other jak inhibitors,.
Food and drug administration (fda) for adults with severe alopecia areata (aa) (1). Web olumiant is indicated for the treatment of adult patients with severe alopecia areata. Web olumiant (baricitinib) is an oral janus kinase (jak) inhibitor approved by the u.s. Ad view prescribing info, safety info & boxed warning. Official patient site for litfulo™. The criteria outlined in this policy is only applicable to coverage in the outpatient setting. Find resources and support for your patients prescribed litfulo®. Not recommended for use in combination with other jak inhibitors,. Ad view prescribing info, safety info & boxed warning. Web “the results of clinical trials are remarkable, as 1 in 5 adults taking olumiant 2mg/day and 1 in 3 taking olumiant 4mg/day achieved significant hair regrowth resulting.
FDA approves Olumiant to treat severe cases of alopecia areata
Web olumiant is a prescription medication that is a janus kinase (jak) inhibitor, and is used to treat adults with severe alopecia areata. Today, history was made as the u.s. Food and drug administration approved olumiant (baricitinib) oral tablets to treat adult patients with severe alopecia areata, a disorder that. Food and drug administration (fda). Under the brand name olumiant.
FDA Approves Eli Lilly's Drug Olumiant For Alopecia Dermatology
Web olumiant (baricitinib) is a new treatment for hair loss due to severe alopecia areata, a condition in which the immune system attacks hair follicles. Web olumiant is indicated for the treatment of adult patients with severe alopecia areata. Official patient site for litfulo™. Web olumiant (baricitinib) is an oral janus kinase (jak) inhibitor approved by the u.s. Ad schedule.
Alopecia Areata Avens Blog Avens Blog
Food and drug administration announced its approval of eli lilly's oral tablet olumiant (baricitinib). Web “the results of clinical trials are remarkable, as 1 in 5 adults taking olumiant 2mg/day and 1 in 3 taking olumiant 4mg/day achieved significant hair regrowth resulting. Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately.
Alopecia Universalis Successfully Treated With Adalimumab Hair
Ad view litfulo™ prescribing info, safety info & boxed warning on the official hcp site. Food and drug administration (fda) for adults with severe alopecia areata (aa) (1). The criteria outlined in this policy is only applicable to coverage in the outpatient setting. Ad schedule a free consultation with one of our hair restoration experts today! Web alopecia areata causes.
Another ‘miracle drug’ for COVID19 increasingly hard to find in
Web olumiant is a prescription medication that is a janus kinase (jak) inhibitor, and is used to treat adults with severe alopecia areata. Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an. Ad view efficacy, safety, full prescribing information, and boxed warning..
Dosing for Rheumatoid Arthritis HCP Olumiant® (baricitinib)
Food and drug administration (fda). Ad view efficacy, safety, full prescribing information, and boxed warning. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant. Not recommended for use in combination with other jak inhibitors,. Web “the results of clinical trials are remarkable, as 1 in 5 adults taking olumiant 2mg/day and 1 in.
Alopecia Areata Treated With Tofacinitib Hair Disorders JAMA
Food and drug administration (fda) for adults with severe alopecia areata (aa) (1). Web olumiant is a prescription medication that is a janus kinase (jak) inhibitor, and is used to treat adults with severe alopecia areata. Web the drug is sold by eli lilly & co. Ad view efficacy, safety, full prescribing information, and boxed warning. Download support resources, including.
FDA approves use of Olumiant to help treat severe alopecia areata
Ad schedule a free consultation with one of our hair restoration experts today! Web the drug is sold by eli lilly & co. Web olumiant (baricitinib) is a new treatment for hair loss due to severe alopecia areata, a condition in which the immune system attacks hair follicles. Ad view efficacy, safety, full prescribing information, and boxed warning. The criteria.
Olumiant es designada Terapia Innovadora por la FDA para el tratamiento
Food and drug administration approved olumiant (baricitinib) oral tablets to treat adult patients with severe alopecia areata, a disorder that. Official patient site for litfulo™. Ad view prescribing info, safety info & boxed warning. Official patient site for litfulo™. Web olumiant is indicated for the treatment of adult patients with severe alopecia areata.
Olumiant offers hope to patients with rheumatoid arthritis Drug
Food and drug administration announced its approval of eli lilly's oral tablet olumiant (baricitinib). Web alopecia areata causes hair loss that can happen unpredictably. Web “the results of clinical trials are remarkable, as 1 in 5 adults taking olumiant 2mg/day and 1 in 3 taking olumiant 4mg/day achieved significant hair regrowth resulting. But some current treatments (though not. Web impairment.
Today, History Was Made As The U.s.
Download support resources, including a doctor discussion guide. Ad view efficacy, safety, full prescribing information, and boxed warning. Web the drug is sold by eli lilly & co. Food and drug administration approved olumiant (baricitinib) oral tablets to treat adult patients with severe alopecia areata, a disorder that.
Under The Brand Name Olumiant And Comes In The Form Of Oral Tablets Taken Once Daily.
Ad schedule a free consultation with one of our hair restoration experts today! Find resources and support for your patients prescribed litfulo®. Web olumiant (baricitinib) is a new treatment for hair loss due to severe alopecia areata, a condition in which the immune system attacks hair follicles. Web olumiant is indicated for the treatment of adult patients with severe alopecia areata.
Download Support Resources, Including A Doctor Discussion Guide.
Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an. Web olumiant (baricitinib) is an oral janus kinase (jak) inhibitor approved by the u.s. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant. Ad view prescribing info, safety info & boxed warning.
Not Recommended For Use In Combination With Other Jak Inhibitors,.
Web impairment (estimated glomerular filtration rate (gfr) between 30 and olumiant</strong> is not recommended. Ad view efficacy, safety, full prescribing information, and boxed warning. Web olumiant is a prescription medication that is a janus kinase (jak) inhibitor, and is used to treat adults with severe alopecia areata. Web june 13, 2022 español today, the u.s.