Ionic Bonds Form Between Atoms With Complementary
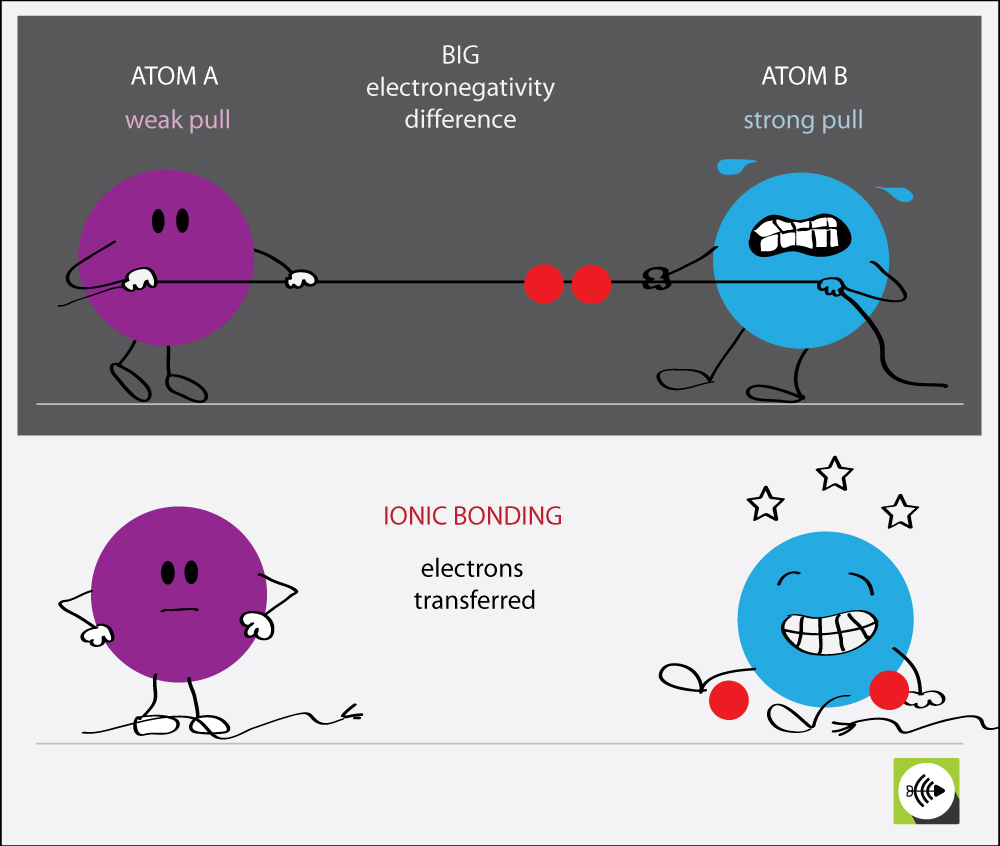
Ionic Bonds Form Between Atoms With Complementary - Introduction living things are made up of atoms, but in most cases, those atoms aren’t just floating around individually. Web ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. In contrast, atoms with the same electronegativity share electrons in covalent bonds, because neither atom preferentially attracts or repels the shared electrons. These ions attract each other. Ions are created when an atom loses or gains an electron. These ions then attract each other electrostatically to form a stable crystalline lattice. Instead, they’re usually interacting with other atoms (or groups of atoms). Electron transfer produces negative ions called anions and positive ions called cations. Web atoms interact with each other through the formation of chemical bonds. An example of a covalent compound is ammonia.
Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. For example, sodium cations (positively charged ions) and chlorine anions (negatively charged ions) are connected via ionic bonds in sodium chloride, or table. Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged ions. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Web compounds can be covalent or ionic. Web types of chemical bonds including covalent, ionic, and hydrogen bonds and london dispersion forces. Web atoms interact with each other through the formation of chemical bonds. These ions then attract each other electrostatically to form a stable crystalline lattice. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Let’s examine the ionic bond in sodium chloride.
Introduction living things are made up of atoms, but in most cases, those atoms aren’t just floating around individually. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Web glossary summary glossary introduction learning objectives explain the formation of cations, anions, and ionic compounds predict the charge of common metallic and nonmetallic elements, and write their electron configurations describe the formation of covalent bonds define electronegativity and assess the polarity of covalent bonds These ions attract each other. One type of chemical bond is an ionic bond. Ionic bonds require at least one electron donor and one electron acceptor. Instead, they’re usually interacting with other atoms (or groups of atoms). These ions then attract each other electrostatically to form a stable crystalline lattice. Web compounds can be covalent or ionic. Let’s examine the ionic bond in sodium chloride.
Examples of Ionic Bonds and Ionic Compounds
Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged ions. One type of chemical bond is an ionic bond. These ions attract each other. Web in ionic bonding, atoms transfer electrons to each other. These ions then attract each other electrostatically to form a.
Chemical Bonds
Introduction living things are made up of atoms, but in most cases, those atoms aren’t just floating around individually. Let’s examine the ionic bond in sodium chloride. These ions attract each other. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Ionic bonds result from the attraction between oppositely charged ions.
Ionic Bonding Presentation Chemistry
One type of chemical bond is an ionic bond. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Web ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. An example of a covalent compound is ammonia. In covalent.
Electronegativity Bond Scale Surfguppy Chemistry made easy for
For example, sodium cations (positively charged ions) and chlorine anions (negatively charged ions) are connected via ionic bonds in sodium chloride, or table. Let’s examine the ionic bond in sodium chloride. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Such a bond forms when the valence (outermost) electrons of one.
Ionic Properties
Electron transfer produces negative ions called anions and positive ions called cations. Ions are created when an atom loses or gains an electron. These ions attract each other. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web ionic bonding is the complete transfer of valence electron (s) between atoms and.
Student Exploration Ionic Bonds Answer Key Quizlet / Ionic Bonds Gizmo
Ions are created when an atom loses or gains an electron. Web compounds can be covalent or ionic. For example, sodium cations (positively charged ions) and chlorine anions (negatively charged ions) are connected via ionic bonds in sodium chloride, or table. Electron transfer produces negative ions called anions and positive ions called cations. These ions attract each other.
Ionic Bond Definition, Types, Properties & Examples
Web in ionic bonding, atoms transfer electrons to each other. Let’s examine the ionic bond in sodium chloride. An example of a covalent compound is ammonia. For example, sodium cations (positively charged ions) and chlorine anions (negatively charged ions) are connected via ionic bonds in sodium chloride, or table. In covalent compounds, atoms form covalent bonds that consist of electron.
Ionic Bond Definition, Types, Properties & Examples
Web in ionic bonding, atoms transfer electrons to each other. Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged ions. One type of chemical bond is an ionic bond. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently.
Chapter 2 Atoms, Molecules and Life Chemistry)
Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web compounds can be covalent or ionic. Ionic bonds require at least one electron donor and one electron acceptor. Web in ionic bonding, atoms transfer electrons to each other. Such a bond forms when the valence (outermost) electrons of one atom are.
이온 성 공유 결합과 금속 결합의 차이점 2021 뉴스
Web compounds can be covalent or ionic. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Ions are created when an atom loses or gains an electron. In covalent compounds, atoms form covalent.
These Ions Attract Each Other.
In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Web ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ions are created when an atom loses or gains an electron. Ionic bonds require at least one electron donor and one electron acceptor.
These Ions Then Attract Each Other Electrostatically To Form A Stable Crystalline Lattice.
One type of chemical bond is an ionic bond. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web in ionic bonding, atoms transfer electrons to each other. Instead, they’re usually interacting with other atoms (or groups of atoms).
Web Atoms Interact With Each Other Through The Formation Of Chemical Bonds.
For example, sodium cations (positively charged ions) and chlorine anions (negatively charged ions) are connected via ionic bonds in sodium chloride, or table. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Electron transfer produces negative ions called anions and positive ions called cations. Web compounds can be covalent or ionic.
Introduction Living Things Are Made Up Of Atoms, But In Most Cases, Those Atoms Aren’t Just Floating Around Individually.
An example of a covalent compound is ammonia. Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Web glossary summary glossary introduction learning objectives explain the formation of cations, anions, and ionic compounds predict the charge of common metallic and nonmetallic elements, and write their electron configurations describe the formation of covalent bonds define electronegativity and assess the polarity of covalent bonds Ionic bonds result from the attraction between oppositely charged ions.
:max_bytes(150000):strip_icc()/ionic-bond-58fd4ea73df78ca1590682ad.jpg)
.PNG)
.PNG)