How Many Covalent Bonds Does Phosphorus Form
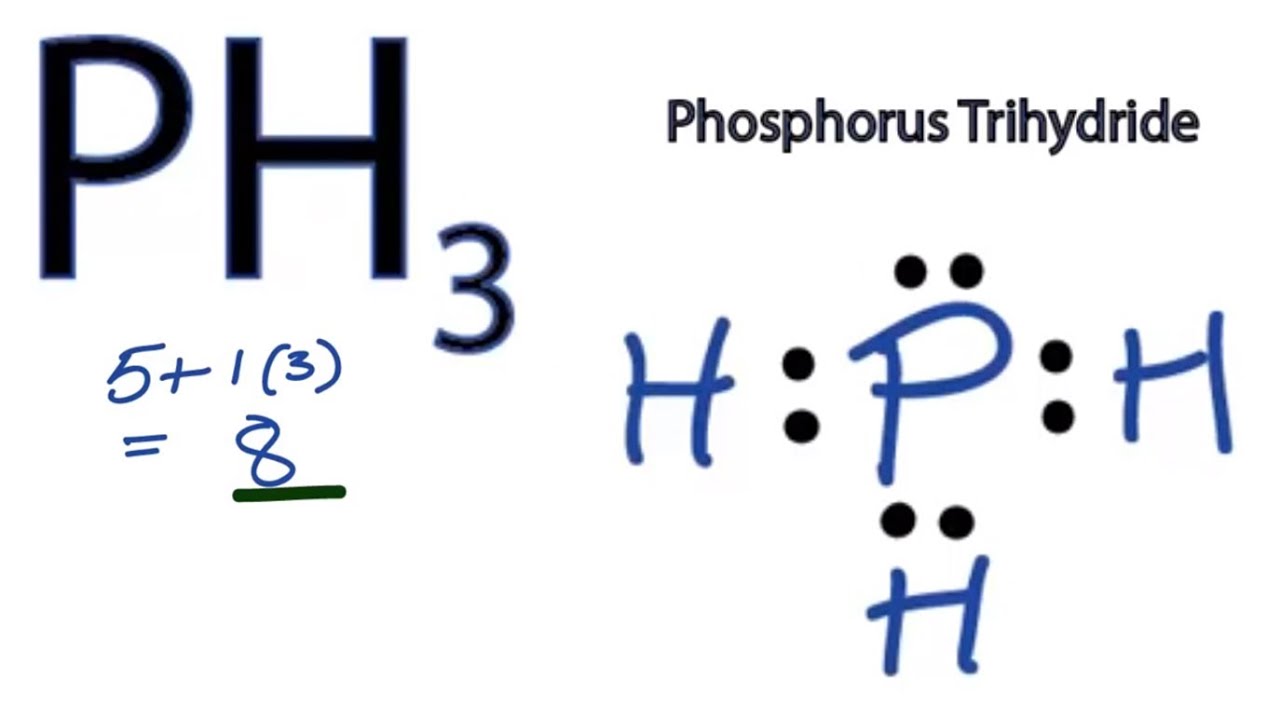
How Many Covalent Bonds Does Phosphorus Form - This is summarized in the table below. Web from the diagram, we can see that phosphorus has a full eight electrons in its outermost shell. Web typically, the atoms of group 4a form 4 covalent bonds; Web correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3. Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web how many bonds does phosphorus typically make? Web how many covalent bonds does a phosphorus atom normally form, based on the number of valence electrons in the atom? Web there are many different modifications of phosphorus in nature. Covalent bond two atoms form a covalent chemical bond. Web the formula of the carbonate ion is co 32−.
Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web from the diagram, we can see that phosphorus has a full eight electrons in its outermost shell. This is summarized in the table below. So there are three covalent bonds and one lone pair of electrons on. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. Covalent bond two atoms form a covalent chemical bond. Group 5a form 3 bonds; And like a central silicon atom, there are four pairs of electrons being shared with. Web there are many different modifications of phosphorus in nature.
And like a central silicon atom, there are four pairs of electrons being shared with. Web some compounds contain both covalent and ionic bonds. Web how many bonds does phosphorus typically make? The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. So, phosphorous can exceed its valency from +3 to +5. Web the formula of the carbonate ion is co 32−. Web there are many different modifications of phosphorus in nature. Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web from the diagram, we can see that phosphorus has a full eight electrons in its outermost shell. Group 5a form 3 bonds;
__TOP__ How Many Covalent Bonds Can Chlorine Form
Web there are many different modifications of phosphorus in nature. Group 5a form 3 bonds; And like a central silicon atom, there are four pairs of electrons being shared with. Web some compounds contain both covalent and ionic bonds. Group 6a form 2 bonds;
Lewis Dot Diagram Phosphorus
Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web typically, the atoms of group 4a form 4 covalent bonds; Group 5a form 3 bonds; Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. So there are three covalent bonds and one.
How Many Single Bonds Can Carbon Form fredhughesdesign
So there are three covalent bonds and one lone pair of electrons on. Web the most commonly observed (formal) oxidation state of phosphorus in molecules and crystals is +3 and +5, although a variety of oxidation states, from −3 to 4,. How many bonds does phosphorus typically make? Web from the diagram, we can see that phosphorus has a full.
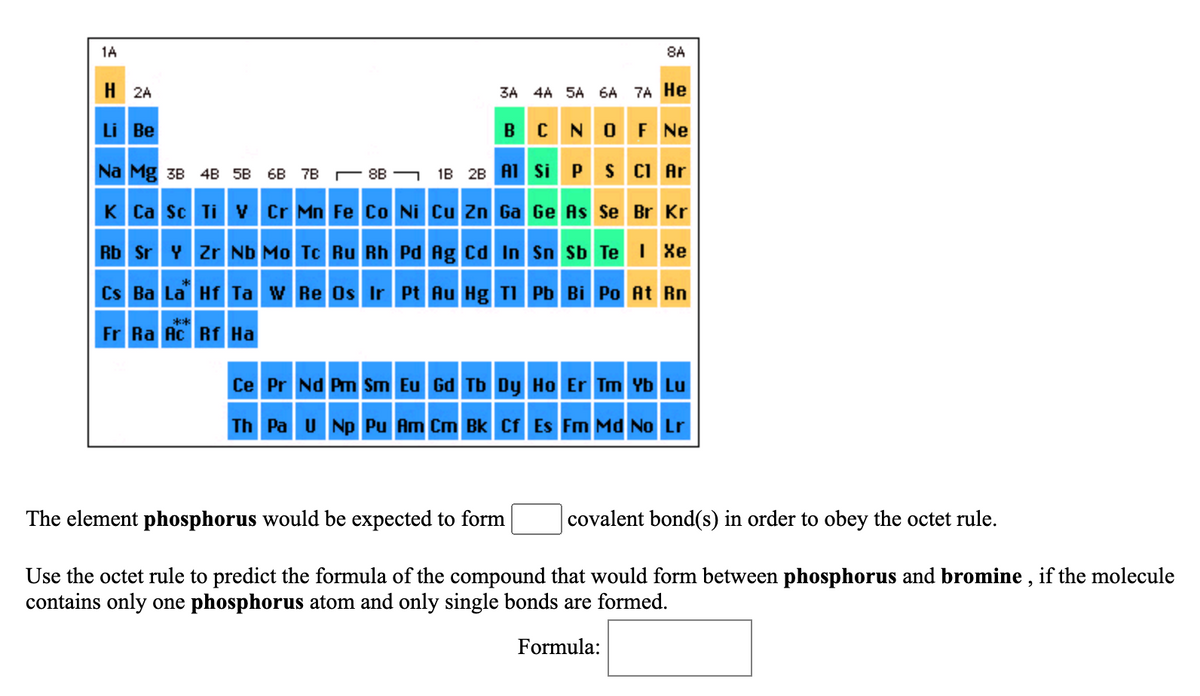
Answered The element phosphorus would be… bartleby
Web from the diagram, we can see that phosphorus has a full eight electrons in its outermost shell. Web typically, the atoms of group 4a form 4 covalent bonds; And group 7a form one bond. Covalent bond two atoms form a covalent chemical bond. Web some compounds contain both covalent and ionic bonds.
FileElectron shell 015 Phosphorus.svg Wikimedia Commons Phosphorus
Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3. Web the formula of the carbonate ion is co 32−. So there are three covalent bonds and one lone pair of electrons.
How does phosphorus form 5 covalent bonds? The Unconditional Guru
Web there are many different modifications of phosphorus in nature. So there are three covalent bonds and one lone pair of electrons on. How many bonds does phosphorus typically make? Web typically, the atoms of group 4a form 4 covalent bonds; Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus.
Is SiO2 Ionic or Covalent? Techiescientist
Web some compounds contain both covalent and ionic bonds. So there are three covalent bonds and one lone pair of electrons on. How many bonds does phosphorus typically make? Web from the diagram, we can see that phosphorus has a full eight electrons in its outermost shell. Group 6a form 2 bonds;
LabXchange
And group 7a form one bond. Group 6a form 2 bonds; So there are three covalent bonds and one lone pair of electrons on. Web the formula of the carbonate ion is co 32−. Web how many covalent bonds does a phosphorus atom normally form, based on the number of valence electrons in the atom?
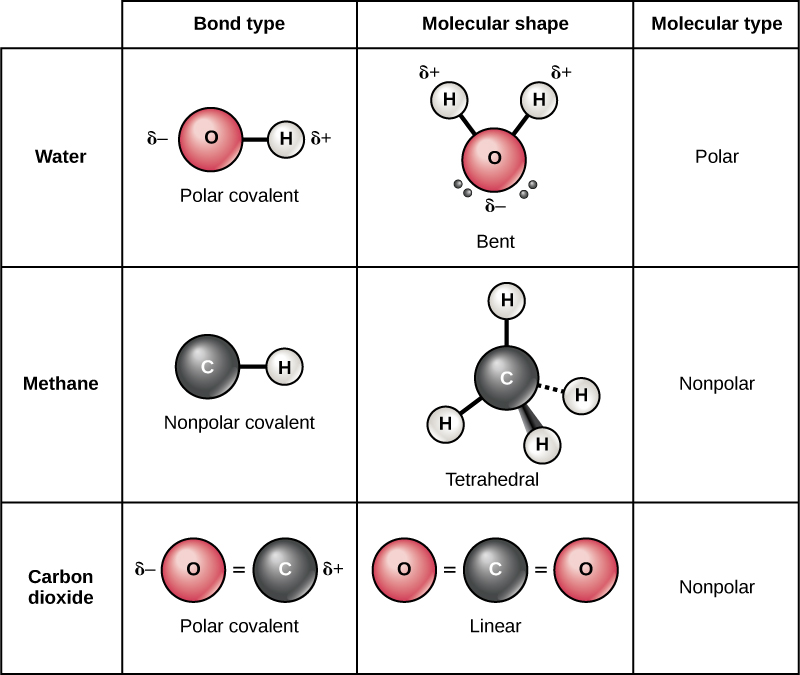
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Group 5a form 3 bonds; So there are three covalent bonds and one lone pair of electrons on. Web typically, the atoms of group 4a form 4 covalent bonds; Group 6a form 2 bonds; Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus.
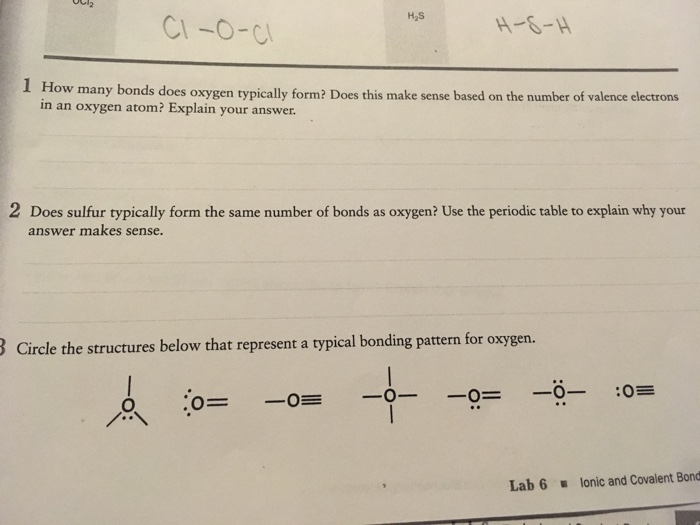
Solved H2S CIOC HSH 1 How many bonds does oxygen
Web the most commonly observed (formal) oxidation state of phosphorus in molecules and crystals is +3 and +5, although a variety of oxidation states, from −3 to 4,. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. This is summarized in the table below. Group 6a form 2 bonds;.
How Many Bonds Does Phosphorus Typically Make?
Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Group 5a form 3 bonds; Web from the diagram, we can see that phosphorus has a full eight electrons in its outermost shell. And like a central silicon atom, there are four pairs of electrons being shared with.
Web How Many Bonds Does Phosphorus Typically Make?
Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web there are many different modifications of phosphorus in nature. Web correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3. Web some compounds contain both covalent and ionic bonds.
The Atoms Of A Polyatomic Ion Are Tightly Bonded Together And So The Entire Ion Behaves As A Single Unit.
So there are three covalent bonds and one lone pair of electrons on. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. And group 7a form one bond. Therefore phosphorus maximum covalency of 6.
So There Are Three Covalent Bonds And One Lone Pair Of Electrons On.
This is summarized in the table below. Web the formula of the carbonate ion is co 32−. Covalent bond two atoms form a covalent chemical bond. Web typically, the atoms of group 4a form 4 covalent bonds;