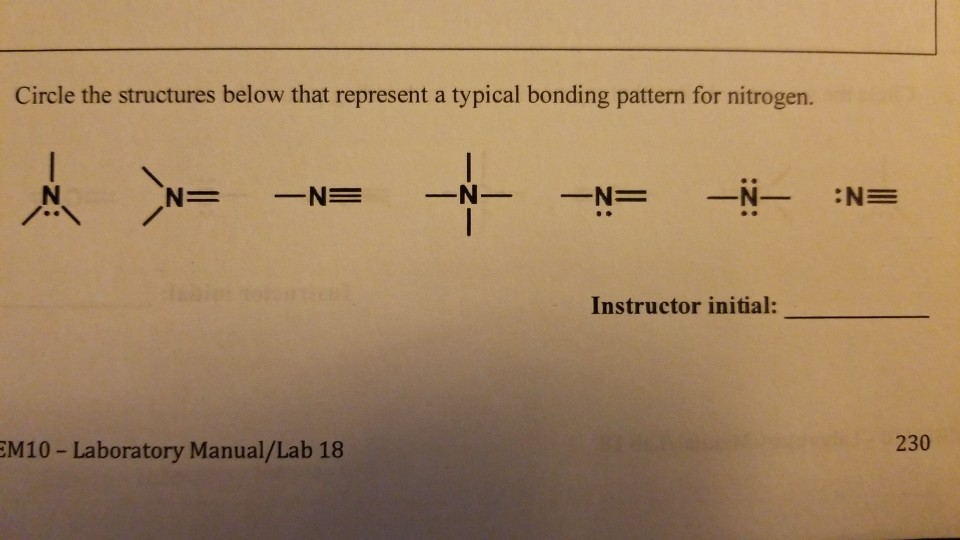
How Many Bonds Can Nitrogen Form
How Many Bonds Can Nitrogen Form - Web the molecule has five bonds and is ‘an extremely stable species’. Through that pair, nitrogen can form an. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom. Examples are nh3 (three single bonds) and n2 (one triple bond). Web two different atoms can also share electrons and form covalent bonds. Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Therefore, it can form three bonds by sharing its three electrons. Web the atom not necessarily has to find another partner to fulfill its valence; Web the number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons). Sometimes nitrogen will form four bonds, like in nh4+, the.
According to the textbooks, a nitrogen atom cannot form more than four bonds. It can even form a bond with itself. Web the number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons). But when we look carefully, we never. Web two different atoms can also share electrons and form covalent bonds. This is because it has atomic number 7, so its electron configuration is 1s22s22p3, giving it 5 valence. Examples are nh3 (three single bonds) and n2 (one triple bond). Therefore, it can form three bonds by sharing its three electrons. Web so if you are following the rules, you might well assume that nitrogen would be able to form five bonds (after all, it has five valence electrons). Web nitrogen has three electrons in its 2p orbital.
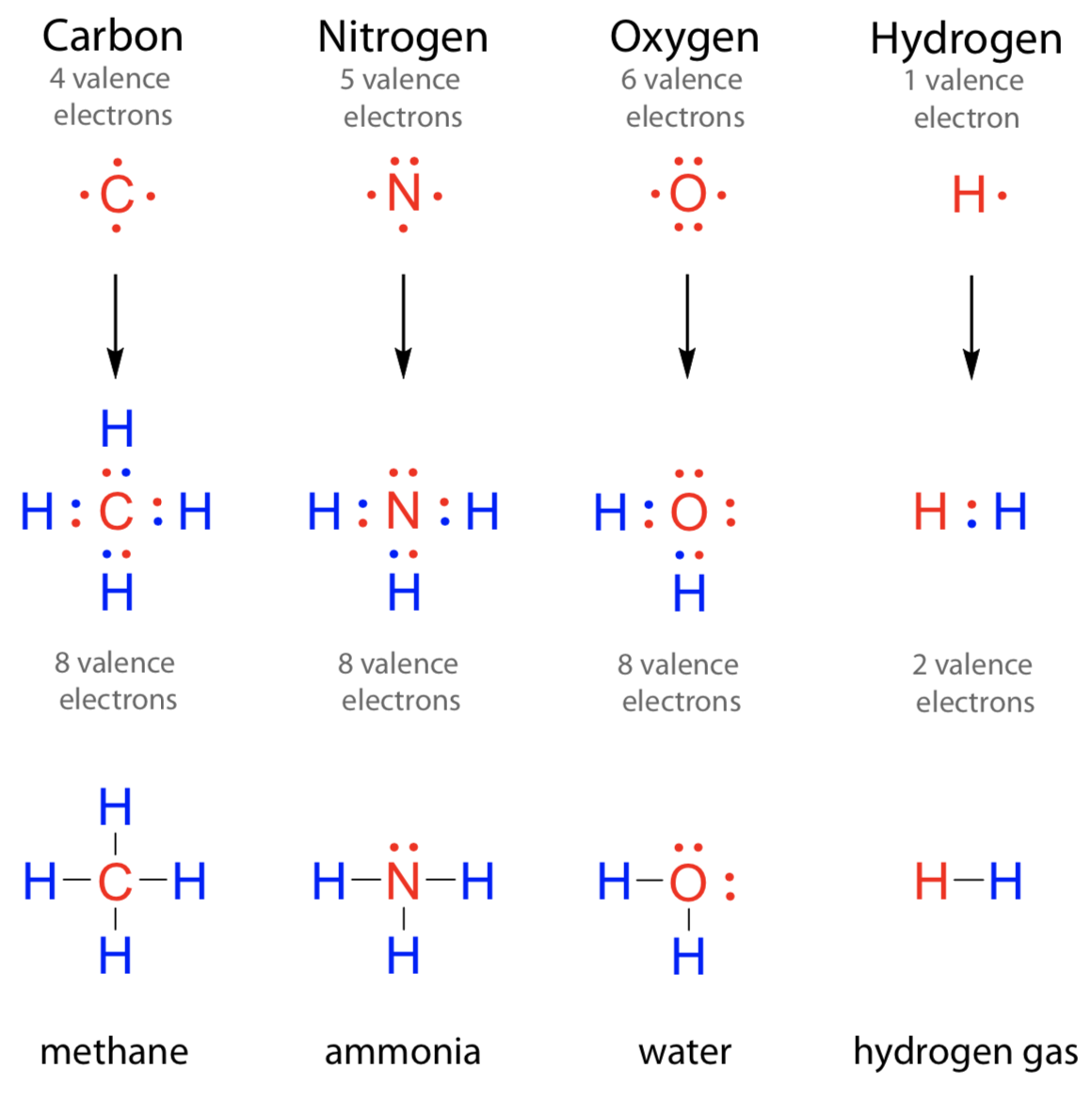
It can even form a bond with itself. According to the textbooks, a nitrogen atom cannot form more than four bonds. Nitrogen atoms donate four electrons to form n 4+ ions and oxygen atoms gain two electrons to form o2− ions when nitrogen and oxygen form an ionic bond. Through that pair, nitrogen can form an. Nitrogen can also have 2 bonds if the nitrogen atom is negatively. This is because it has atomic number 7, so its electron configuration is 1s22s22p3, giving it 5 valence. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Web nitrogen has three electrons in its 2p orbital. Sometimes nitrogen will form four bonds, like in nh4+, the.
Nitrogen Definition, Symbol, Uses, Properties, Atomic Number, & Facts
Web nitrogen typically forms 3 covalent bonds, including in n 2. For example, oxygen has a valency of two and Web nitrogen will usually have 3 bonds, occasionally 4; Web two different atoms can also share electrons and form covalent bonds. Examples are nh3 (three single bonds) and n2 (one triple bond).
[Solved] How many bonds can nitrogen form? 9to5Science
Web the atom not necessarily has to find another partner to fulfill its valence; Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Group 5a (15) elements such as nitrogen. Examples are nh3 (three single bonds) and n2 (one triple bond). Through that pair, nitrogen can form an.
Solved How many bonds does nitrogen typically form? Does
It can even form a bond with itself. Sometimes nitrogen will form four bonds, like in nh4+, the. According to the textbooks, a nitrogen atom cannot form more than four bonds. Examples are nh3 (three single bonds) and n2 (one triple bond). Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in.
How Many Bonds Can Nitrogen Form Jacks Of Science
Web nitrogen will usually have 3 bonds, occasionally 4; It can even form a bond with itself. Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Sometimes nitrogen will form four bonds, like in nh4+, the. According to the textbooks, a nitrogen atom cannot form more than four bonds.
How Many Bonds Can Nitrogen Form Jacks Of Science
Web the atom not necessarily has to find another partner to fulfill its valence; Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). According to the textbooks, a nitrogen atom cannot form more than four bonds. For example, oxygen has a valency of two and Nitrogen is a.
LabXchange
Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). For example, oxygen has a valency of two and According to the textbooks, a nitrogen.
LabXchange
Nitrogen atoms donate four electrons to form n 4+ ions and oxygen atoms gain two electrons to form o2− ions when nitrogen and oxygen form an ionic bond. Web the number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons). However, if the n has.
How Many Bonds Can Nitrogen Form Jacks Of Science
Examples are nh3 (three single bonds) and n2 (one triple bond). But when we look carefully, we never. This is because it has atomic number 7, so its electron configuration is 1s22s22p3, giving it 5 valence. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images.
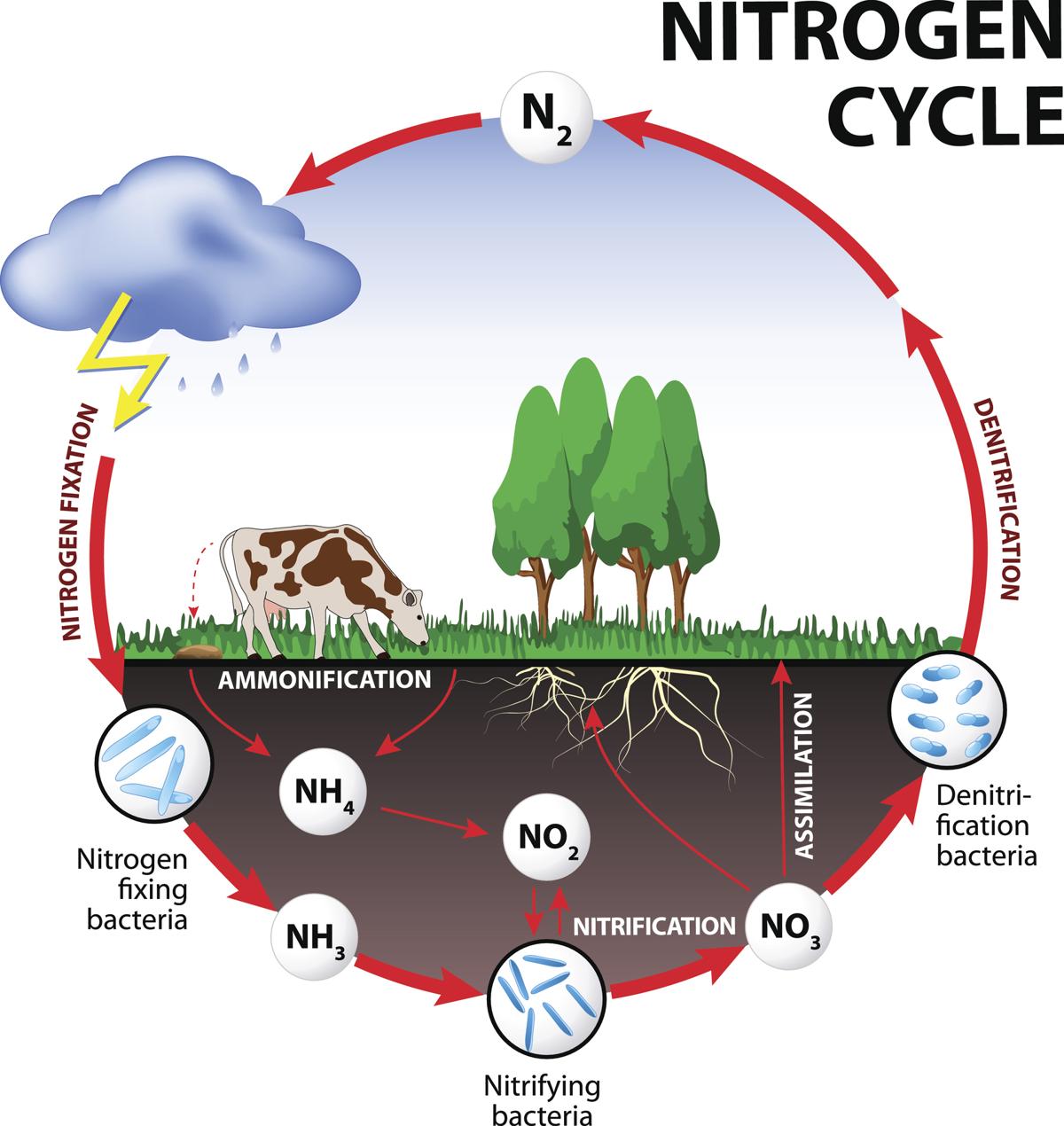
Nitrogen cycle Steps of Nitrogen cycle Online Biology Notes
For example, oxygen has a valency of two and Web so if you are following the rules, you might well assume that nitrogen would be able to form five bonds (after all, it has five valence electrons). Web two different atoms can also share electrons and form covalent bonds. It can even form a bond with itself. According to the.
How Many Bonds Does Nitrogen Form tour bous
For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom. Web two different atoms can also share electrons and form covalent bonds. Examples are nh3 (three single bonds) and n2 (one triple bond). Through that pair, nitrogen can form an. According to the textbooks, a nitrogen atom cannot form more than four bonds.
Web The Atom Not Necessarily Has To Find Another Partner To Fulfill Its Valence;
Web the molecule has five bonds and is ‘an extremely stable species’. Web nitrogen has three electrons in its 2p orbital. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). It can even form a bond with itself.
Web Two Different Atoms Can Also Share Electrons And Form Covalent Bonds.
For example, oxygen has a valency of two and This is because it has atomic number 7, so its electron configuration is 1s22s22p3, giving it 5 valence. Web the number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons). Through that pair, nitrogen can form an.
Nitrogen Is A Colourless, Odourless Gas, Which Condenses At −195.8 °C To A Colourless, Mobile Liquid.
According to the textbooks, a nitrogen atom cannot form more than four bonds. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom. Web nitrogen will usually have 3 bonds, occasionally 4; Web nitrogen typically forms 3 covalent bonds, including in n 2.
Therefore, It Can Form Three Bonds By Sharing Its Three Electrons.
Nitrogen atoms donate four electrons to form n 4+ ions and oxygen atoms gain two electrons to form o2− ions when nitrogen and oxygen form an ionic bond. But when we look carefully, we never. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Group 5a (15) elements such as nitrogen.