How Do Metals Form Ions
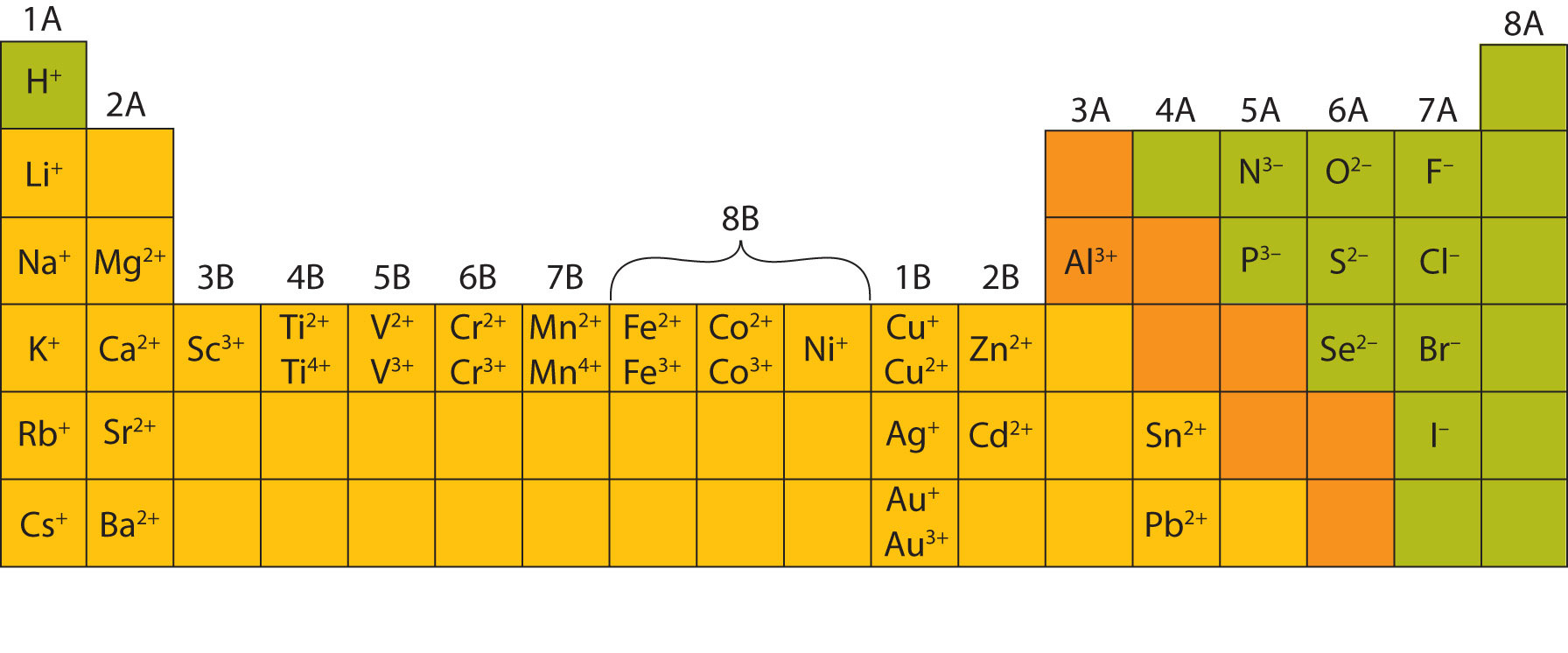
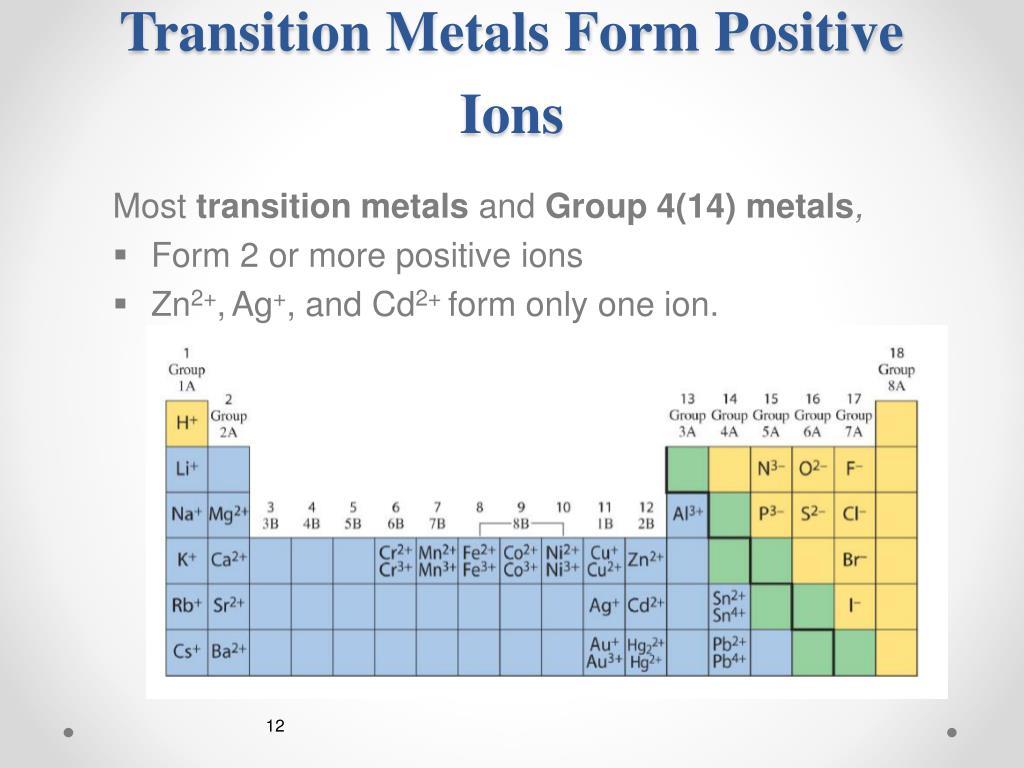
How Do Metals Form Ions - An iron (ii) ion has a 2+ charge, and an iron (iii) ion has a 3+ charge. Web metal ions in aqueous solution. Many transition metals can form more than one ion. Metals tend to form cations, while nonmetals tend to form anions. The number lost is the same as. Web this is actually one of the chemical properties of metals and nonmetals: Ions form primarily through loss of \(s\) electrons. Web how do metals form ions? Second, most atoms form ions of a single. Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic configuration, as most metals have electrons.
Web ions form when atoms lose or gain electrons. Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic configuration, as most metals have electrons. Ions form primarily through loss of \(s\) electrons. By combination of ions with other. Metals form electopositive ions or cations. Web ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; Web transition metals have unfilled inner \(d\) electron shells. It is formed in the way that all minerals generally are. A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m (h 2 o) n] z+. Web you’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges.
Web how do minerals form from water solutions? The number lost is the same as. Metals form electopositive ions or cations. By combination of ions with other. Web ions are formed when atoms lose or gain negatively charged electrons. Web this is actually one of the chemical properties of metals and nonmetals: It is formed in the way that all minerals generally are. Second, most atoms form ions of a single. Second, most atoms form ions of a single. Web ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions;
Ions
Second, most atoms form ions of a single. Second, most atoms form ions of a single. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. Ions form primarily through loss of \(s\) electrons. There are probably more ways to form minerals than there are types of minerals themselves.
CH105 Chapter 3 Ionic and Covelent Bonding Chemistry
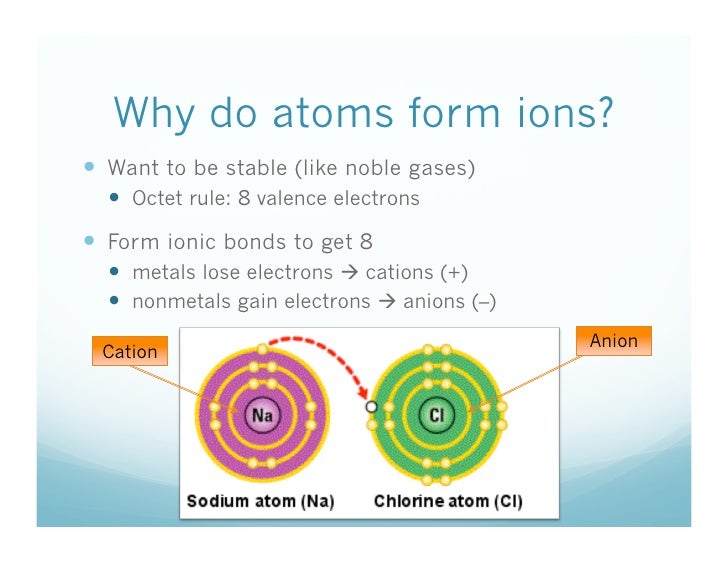
Metals tend to form cations, while nonmetals tend to form anions. Metals form electopositive ions or cations. Ions form primarily through loss of \(s\) electrons. Web this is actually one of the chemical properties of metals and nonmetals: Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas.
Chem matters ch6_ionic_bond
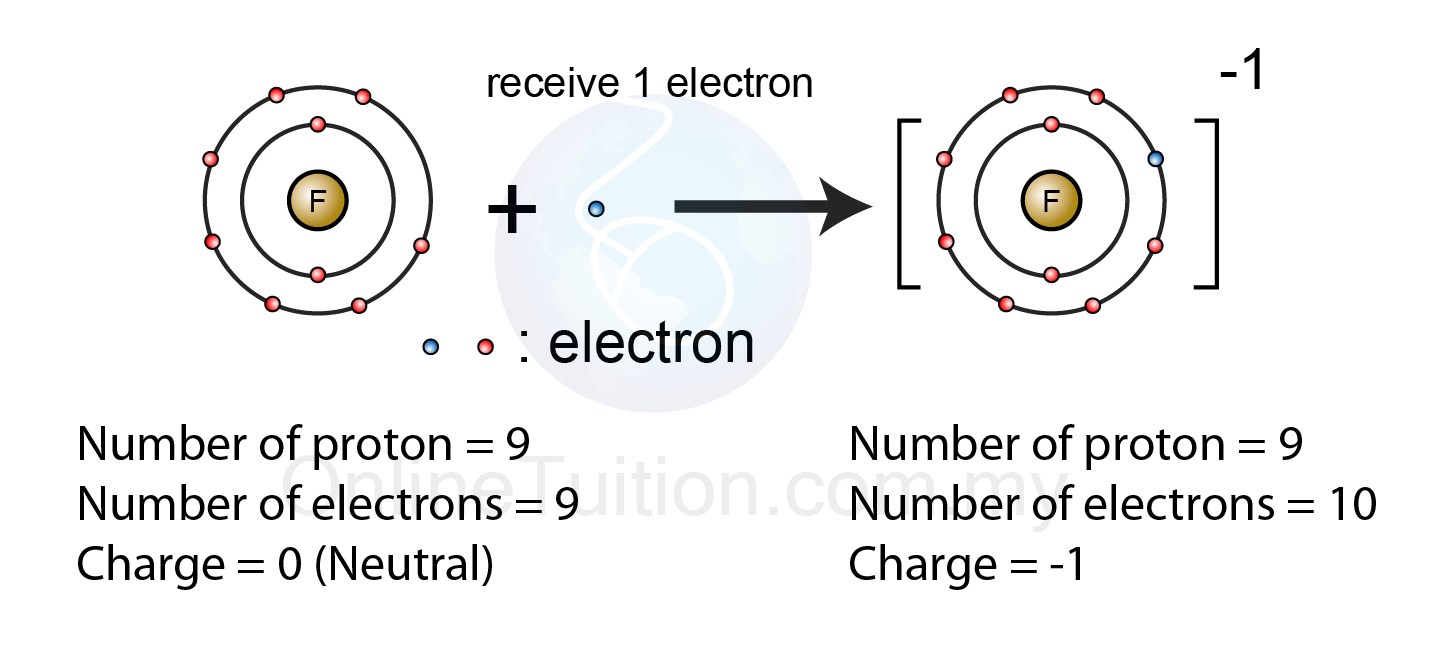
The number of electrons lost or gained determines the charge of the ion: Web how do minerals form from water solutions? Web minerals are found on the earth's crust and mantle and many were created when feldspar and quartz reacted with other materials during the formation of the planet. The number lost is the same as. By combination of ions.
Chem matters ch6_ionic_bond
Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. The number of electrons lost or gained determines the charge of the ion: Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic configuration, as most metals have electrons..
5.2.1 Formation of Ion Revision.my
Web how do metals form ions? Web ions are formed when atoms lose or gain negatively charged electrons. The number lost is the same as. Web this is actually one of the chemical properties of metals and nonmetals: Web the minerals in the original rock were formed at one set of conditions, but were then subjected to different conditions of.
PPT 1 Name the ions formed by these elements and classify them as
A geode is a rounded, hollow rock that is often lined with mineral crystals. Web the minerals in the original rock were formed at one set of conditions, but were then subjected to different conditions of heat, pressure, and h 2 o abundance in earth’s crust. Web this is actually one of the chemical properties of metals and nonmetals: Web.
Write My Paper For Me how to write ions
By combination of ions with other. Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic configuration, as most metals have electrons. There are probably more ways to form minerals than there are types of minerals themselves. Second, most atoms form ions of a single. It contains.
PPT Naming Ionic Compounds PowerPoint Presentation, free download
By combination of ions with other. There are probably more ways to form minerals than there are types of minerals themselves. Ions form primarily through loss of \(s\) electrons. Metals tend to form cations, while nonmetals tend to form anions. Granite is a type of rock that forms from magma.
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge
Web transition metals have unfilled inner \(d\) electron shells. Many transition metals can form more than one ion. It contains the minerals quartz (clear), plagioclase feldspar (shiny white), potassium feldspar (pink), and. Web minerals are found on the earth's crust and mantle and many were created when feldspar and quartz reacted with other materials during the formation of the planet..
PPT How do atoms form ions? PowerPoint Presentation ID7021047
Web ions are formed when atoms lose or gain negatively charged electrons. Web this is actually one of the chemical properties of metals and nonmetals: Metals tend to form cations, while nonmetals tend to form anions. Web ions form when atoms lose or gain electrons. Web this is actually one of the chemical properties of metals and nonmetals:
Metals Tend To Form Cations, While Nonmetals Tend To Form Anions.
Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic configuration, as most metals have electrons. Granite is a type of rock that forms from magma. A geode is a rounded, hollow rock that is often lined with mineral crystals. Web minerals are found on the earth's crust and mantle and many were created when feldspar and quartz reacted with other materials during the formation of the planet.
Many Transition Metals Can Form More Than One Ion.
A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m (h 2 o) n] z+. Metals form electopositive ions or cations. Web the minerals in the original rock were formed at one set of conditions, but were then subjected to different conditions of heat, pressure, and h 2 o abundance in earth’s crust. Web this is actually one of the chemical properties of metals and nonmetals:
It Contains The Minerals Quartz (Clear), Plagioclase Feldspar (Shiny White), Potassium Feldspar (Pink), And.
Web metal ions in aqueous solution. Metals tend to form cations, while nonmetals tend to form anions. Web ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; To obtain a full outer shell:
Web This Is Actually One Of The Chemical Properties Of Metals And Nonmetals:
Web ions are formed when atoms lose or gain negatively charged electrons. Web ions form when atoms lose or gain electrons. An iron (ii) ion has a 2+ charge, and an iron (iii) ion has a 3+ charge. The number of electrons lost or gained determines the charge of the ion: