How Do Ionic Crystals Form
How Do Ionic Crystals Form - Web ionic crystals [in chemistry, an ionic crystal is a crystalline form of an ionic compound. Web ionic compounds form when atoms connect to one another by ionic bonds. Encyclopedia of physical science and technology (third. The arrangement maximizes the attractive force between oppositely. An ionic bond is the strongest type of chemical bond, which leads to characteristic. Web metals and ionic compounds typically form ordered, crystalline solids. Ionic crystals consist of alternating cations and anions held together by electrostatic forces. Web ionic crystals are crystalline structures that grow from ionic bonds and are held together by electrostatic attraction. Ionic bonds are atomic bonds created by the. Their arrangement varies depending on the ions' sizes or the radius ratio (the ratio of the radii of the positive.
Web metals and ionic compounds typically form ordered, crystalline solids. Web ionic compounds form when atoms connect to one another by ionic bonds. Encyclopedia of physical science and technology (third. They are solids consisting of ions bound together by their electrostatic. Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. An ionic bond is the strongest type of chemical bond, which leads to characteristic. Web how are crystals formed in ionic chemistry? The ions may either be. They are solids consisting of ions bound together by their electrostatic attraction into a regular lattice. Metal ions in the alkaline earth series (magnesium [mg], calcium.
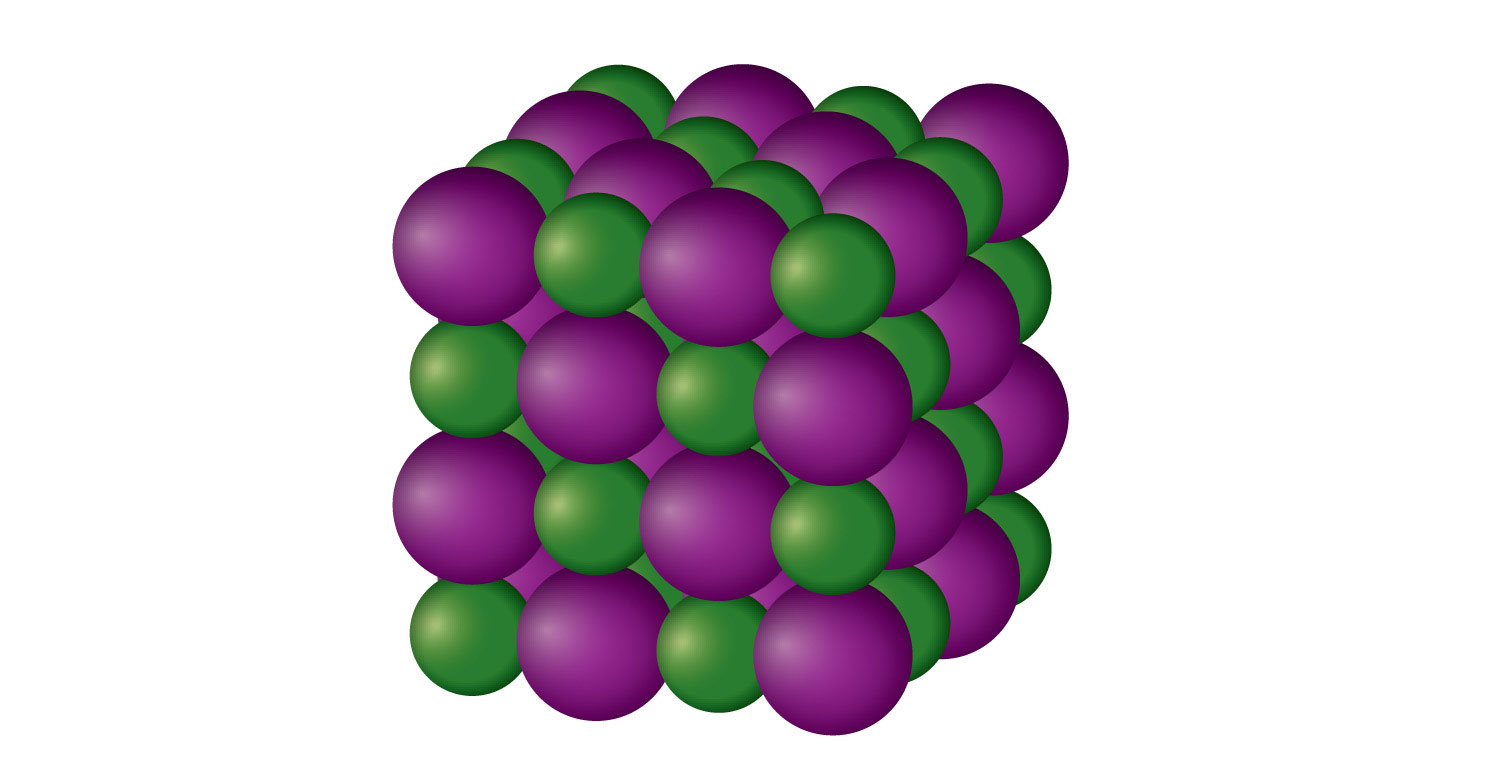
The ions may either be. They are solids consisting of ions bound together by their electrostatic. The arrangement maximizes the attractive force between oppositely. Web ionic crystals [in chemistry, an ionic crystal is a crystalline form of an ionic compound. Metal ions in the alkaline earth series (magnesium [mg], calcium. The crystals have ionic bonding, and each ion has six or eight neighbours. Ionic bonds are atomic bonds created by the. Web ions bound together by electrostatic attraction form ionic crystals. Web metals and ionic compounds typically form ordered, crystalline solids. Web ionic crystals form as negative ions and positive ions electrostatically attracted to each other come together, forming a structure of alternating cations and.
Ionic Bonding Presentation Chemistry
Substances that consist of large molecules, or a mixture of molecules whose movements are more. The arrangement maximizes the attractive force between oppositely. Web ionic compounds form when atoms connect to one another by ionic bonds. Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. An ionic bond is the strongest type of chemical bond,.
Ionic Crystals Introduction to Chemistry
Metal ions in the alkaline earth series (magnesium [mg], calcium. Web ions bound together by electrostatic attraction form ionic crystals. Encyclopedia of physical science and technology (third. Web ionic crystals [in chemistry, an ionic crystal is a crystalline form of an ionic compound. Web metals and ionic compounds typically form ordered, crystalline solids.
Ionic compound wikidoc
Web ionic compounds form when atoms connect to one another by ionic bonds. Web i'm in general chemistry, and i was wondering how ionic species crystallize and form the shapes they do, specifically in regard to the struvite precipitate. Metal ions in the alkaline earth series (magnesium [mg], calcium. Web ionic crystals [in chemistry, an ionic crystal is a crystalline.
Comparison Between Metallic & Ionic Crystals Sciencing
Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. Web i'm in general chemistry, and i was wondering how ionic species crystallize and form the shapes they do, specifically in regard to the struvite precipitate. The ions may either be. Web ions bound together by electrostatic attraction form ionic crystals. Ionic crystals consist of alternating.
10.5 Solids Chemistry LibreTexts
Web ionic crystals form as negative ions and positive ions electrostatically attracted to each other come together, forming a structure of alternating cations and. Metal ions in the alkaline earth series (magnesium [mg], calcium. In this experiment, we will form crystals of two compounds:. Substances that consist of large molecules, or a mixture of molecules whose movements are more. Their.
Ionic Crystals Introduction to Chemistry
The ions may either be. The arrangement maximizes the attractive force between oppositely. Encyclopedia of physical science and technology (third. Web ionic crystals form as negative ions and positive ions electrostatically attracted to each other come together, forming a structure of alternating cations and. An ionic bond is the strongest type of chemical bond, which leads to characteristic.
Ionic Bond Definition Easy Hard Science
They are solids consisting of ions bound together by their electrostatic. Metal ions in the alkaline earth series (magnesium [mg], calcium. In this experiment, we will form crystals of two compounds:. The ions may either be. Web ionic crystals form as negative ions and positive ions electrostatically attracted to each other come together, forming a structure of alternating cations and.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
An ionic bond is the strongest type of chemical bond, which leads to characteristic. They are solids consisting of ions bound together by their electrostatic attraction into a regular lattice. The ions may either be. Web ionic compounds form when atoms connect to one another by ionic bonds. Web ionic crystals [in chemistry, an ionic crystal is a crystalline form.
What Is An Ionic Compound? Formula and Defination
Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. Web i'm in general chemistry, and i was wondering how ionic species crystallize and form the shapes they do, specifically in regard to the struvite precipitate. Metal ions in the alkaline earth series (magnesium [mg], calcium. Encyclopedia of physical science and technology (third. Ionic crystals consist.
7.1 Comparing Ionic and Molecular Substances Chemistry LibreTexts
They are solids consisting of ions bound together by their electrostatic attraction into a regular lattice. Web ionic compounds form when atoms connect to one another by ionic bonds. The arrangement maximizes the attractive force between oppositely. Web ionic crystals [in chemistry, an ionic crystal is a crystalline form of an ionic compound. Ionic crystals consist of alternating cations and.
Web Ionic Crystals [In Chemistry, An Ionic Crystal Is A Crystalline Form Of An Ionic Compound.
Their arrangement varies depending on the ions' sizes or the radius ratio (the ratio of the radii of the positive. Web ionic compounds form when atoms connect to one another by ionic bonds. Ionic bonds are atomic bonds created by the. Web metals and ionic compounds typically form ordered, crystalline solids.
Web Ions Bound Together By Electrostatic Attraction Form Ionic Crystals.
Web ionic crystals are crystalline structures that grow from ionic bonds and are held together by electrostatic attraction. The arrangement maximizes the attractive force between oppositely. Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. An ionic bond is the strongest type of chemical bond, which leads to characteristic.
Web I'm In General Chemistry, And I Was Wondering How Ionic Species Crystallize And Form The Shapes They Do, Specifically In Regard To The Struvite Precipitate.
Web how are crystals formed in ionic chemistry? Web ionic crystals form as negative ions and positive ions electrostatically attracted to each other come together, forming a structure of alternating cations and. Substances that consist of large molecules, or a mixture of molecules whose movements are more. Ionic crystals consist of alternating cations and anions held together by electrostatic forces.
Metal Ions In The Alkaline Earth Series (Magnesium [Mg], Calcium.
They are solids consisting of ions bound together by their electrostatic. In this experiment, we will form crystals of two compounds:. The crystals have ionic bonding, and each ion has six or eight neighbours. The ions may either be.
.PNG)





.PNG)

