Does Sulfur And Barium Form An Ionic Compound
Does Sulfur And Barium Form An Ionic Compound - Other sulfides of barium are ba 2 s, bas 2 , and. Web the two ions are bound trough an ionic bond. Web write formulas for the binary ionic compounds formed between the following pairs of elements: The barium sulfide chemical formula is bas. Web barium and sulfur form several sulfides, of which the most studied is barium sulfide (bas) (81.07% ba), which melts at 2200°c. Web to find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Its chemical structure can be. In its compounds strontium has an exclusive oxidation state of +2, as the sr 2+ ion. (a) given element is barium = symbol is ( ba ) sulfur symbol is ( s ) we know that ionic compound formed from ions cation and anion cations are ( + ve ) charged. Web the metal is readily attacked by acids.
Barium sulfide is the inorganic compound with the formula bas. Sulfuric acid is a notable exception because passivation stops the reaction by forming the insoluble barium sulfate on the surface. Web barium and sulfur form several sulfides, of which the most studied is barium sulfide (bas) (81.07% ba), which melts at 2200°c. (a) given element is barium = symbol is ( ba ) sulfur symbol is ( s ) we know that ionic compound formed from ions cation and anion cations are ( + ve ) charged. It is an important precursor to other barium. The barium sulfide chemical formula is bas. Then, identify the anion and write down its symbol and charge. Its chemical structure can be. Web what is the formula of the ionic compound formed by barium and sulfur? In general, the chemistry of strontium is quite similar to that of calcium.
Web the formula bas is barium sulfide.barium sulfide is an inorganic compound, and an important precursor to other barium compounds.ba = bariums =. Other sulfides of barium are ba 2 s, bas 2 , and. Web write formulas for the binary ionic compounds formed between the following pairs of elements: Bas is the barium compound produced on the. Web barium and sulfur form several sulfides, of which the most studied is barium sulfide (bas) (81.07% ba), which melts at 2200°c. Na 2 o (sodium oxide) na 2 s (sodium sulfide) h 2 o (water) h 2 s (hydrogen sulfide) o 3 (ozone) so 2 (sulfur dioxide) co 2 (carbon. (a) given element is barium = symbol is ( ba ) sulfur symbol is ( s ) we know that ionic compound formed from ions cation and anion cations are ( + ve ) charged. Web the two ions are bound trough an ionic bond. In general, the chemistry of strontium is quite similar to that of calcium. Then, identify the anion and write down its symbol and charge.
Solved What is the formula for an ionic compound that
Barium sulfide is the inorganic compound with the formula bas. Web the two ions are bound trough an ionic bond. It is an important precursor to other barium. Other sulfides of barium are ba 2 s, bas 2 , and. Sulfuric acid is a notable exception because passivation stops the reaction by forming the insoluble barium sulfate on the surface.
Is Ba(OH)2 (Barium hydroxide) Ionic or Covalent? YouTube
Other sulfides of barium are ba 2 s, bas 2 , and. Web to find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Barium sulfide is the inorganic compound with the formula bas. Web the formula bas is barium sulfide.barium sulfide is an inorganic compound, and an important precursor to other.
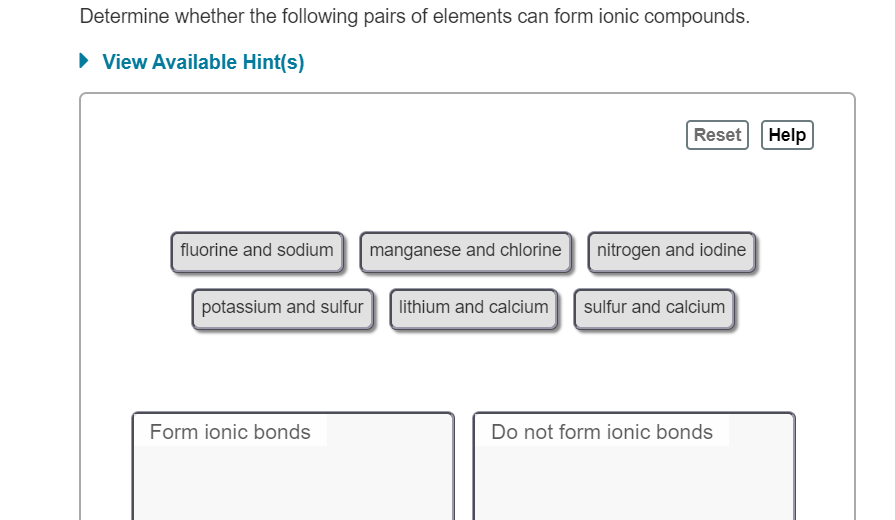
Solved Determine Whether the following pairs of elements can
Web barium and sulfur form several sulfides, of which the most studied is barium sulfide (bas) (81.07% ba), which melts at 2200°c. Sulfuric acid is a notable exception because passivation stops the reaction by forming the insoluble barium sulfate on the surface. Web barium sulfide is also known to undergo slow oxidation in solution to form elemental sulfur and various.
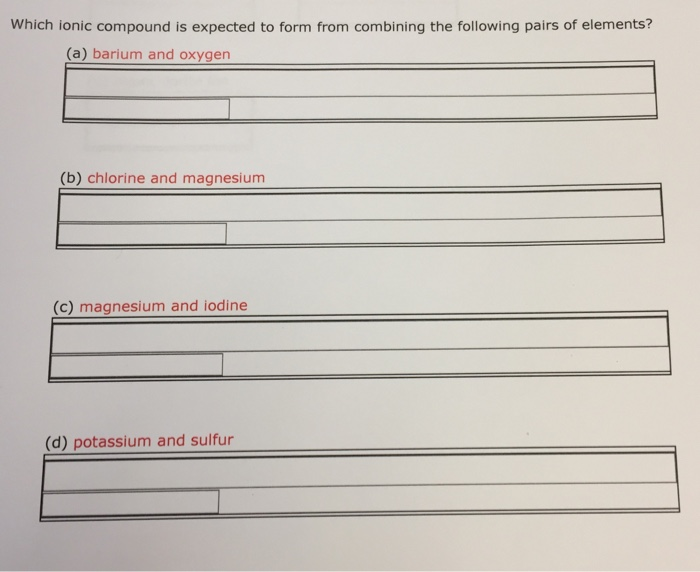
Solved Which ionic compound is expected to form from
Web barium and sulfur form several sulfides, of which the most studied is barium sulfide (bas) (81.07% ba), which melts at 2200°c. It is an important precursor to other barium. Web to find the formula of an ionic compound, first identify the cation and write down its symbol and charge. The geometry of the molecule is octahedral with one cation.
does barium and rubidium form an ionic compound (2023)
Web the two ions are bound trough an ionic bond. Web barium sulfide is the inorganic compound with the formula ba s. Barium sulfide is the inorganic compound with the formula bas. Web barium sulfide is also known to undergo slow oxidation in solution to form elemental sulfur and various oxidized sulfur species including the sulfite, thiosulfate, polythionates, and. Then,.
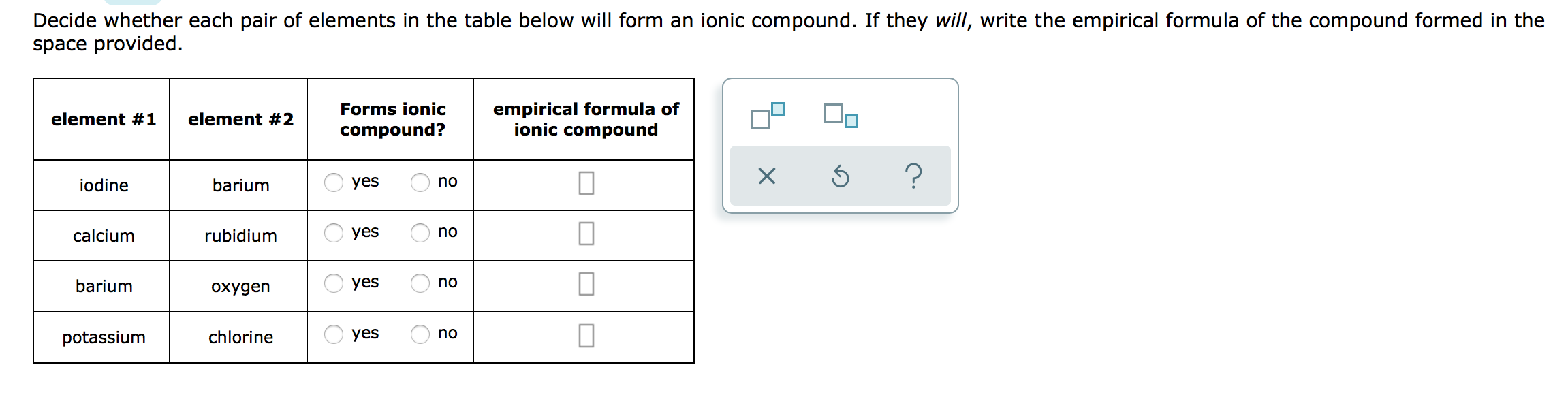
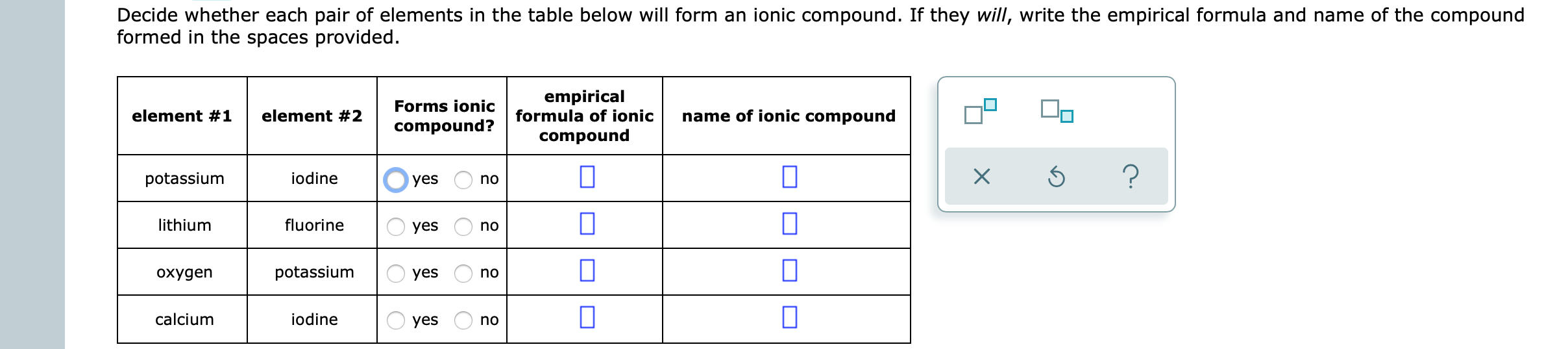
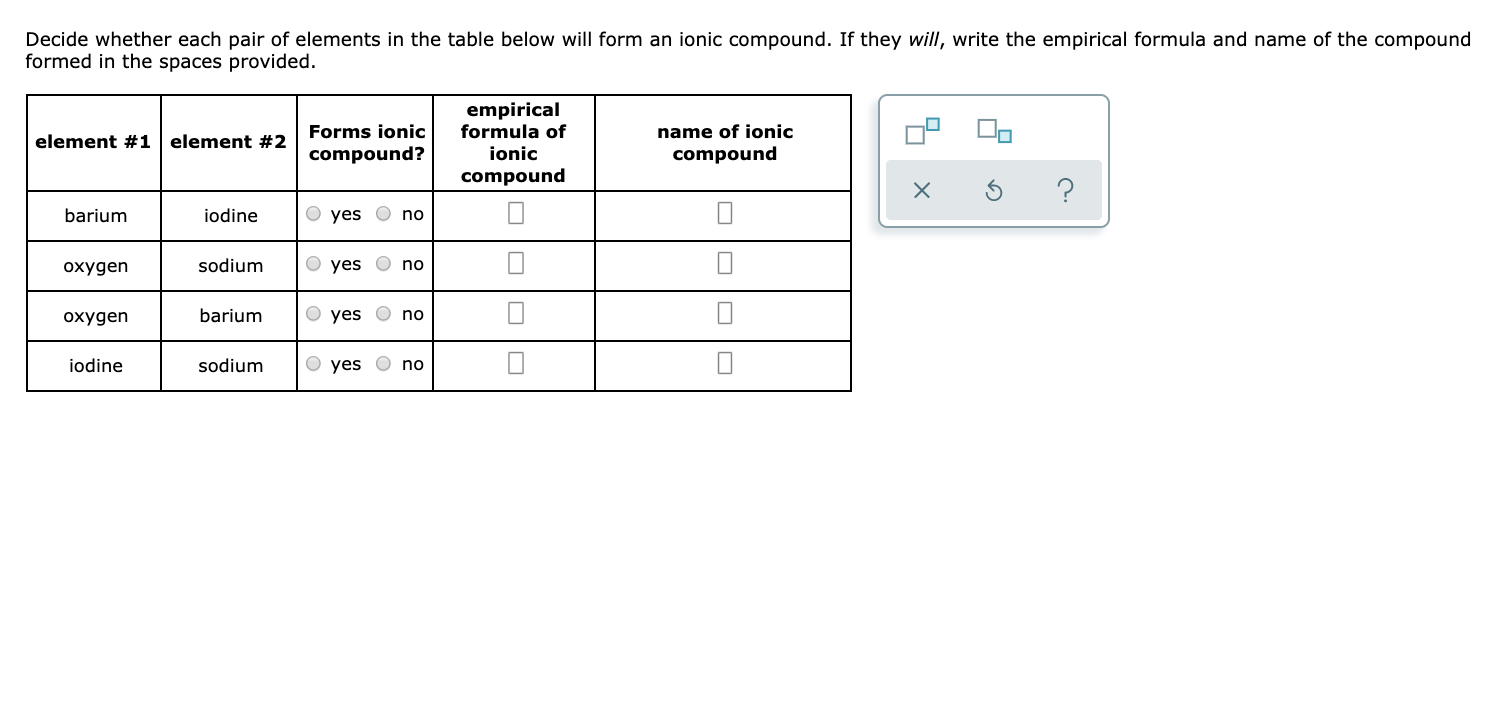
Solved Decide whether each pair of elements in the table
The geometry of the molecule is octahedral with one cation surrounded by eight anions and vice versa. Web sulfur barium ionic compound. Then, identify the anion and write down its symbol and charge. It is an important precursor to other barium. Web the two ions are bound trough an ionic bond.
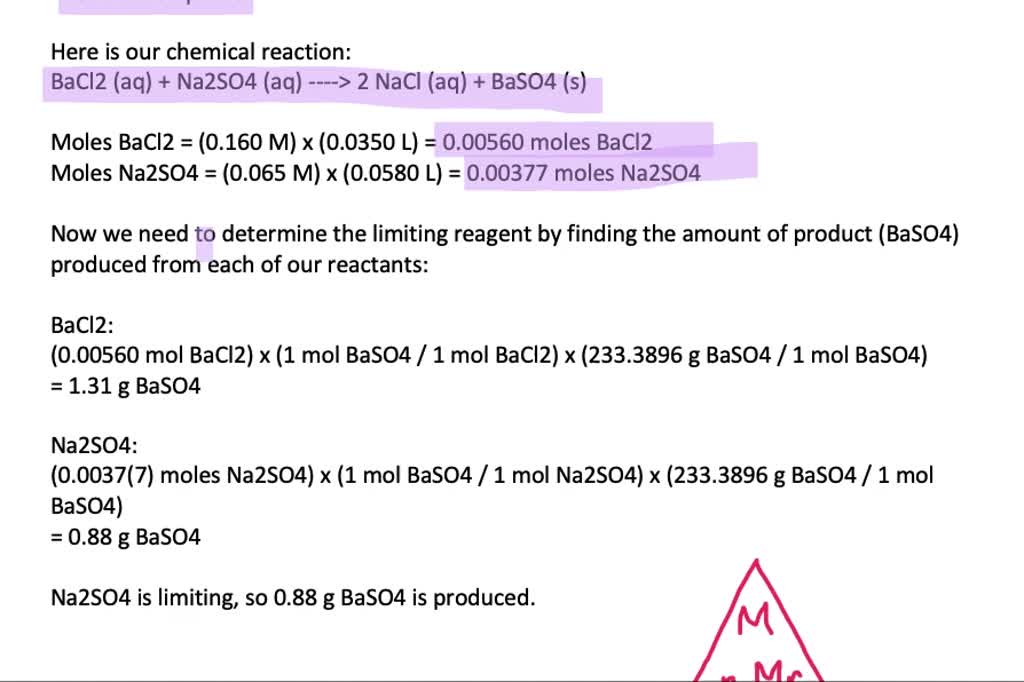
SOLVEDHow many grams of barium sulfate form when 35.0 mL of 0.160 M
Web barium sulfide is also known to undergo slow oxidation in solution to form elemental sulfur and various oxidized sulfur species including the sulfite, thiosulfate, polythionates, and. Other sulfides of barium are ba 2 s, bas 2 , and. Its chemical structure can be. Bas is the barium compound produced on the largest scale. In its compounds strontium has an.
Answered Decide whether each pair of elements in… bartleby
(a) given element is barium = symbol is ( ba ) sulfur symbol is ( s ) we know that ionic compound formed from ions cation and anion cations are ( + ve ) charged. Web what is the formula of the ionic compound formed by barium and sulfur? Web to find the formula of an ionic compound, first identify.
Solved When solutions of ammonium sulfate and barium
Barium sulfide is the inorganic compound with the formula bas. Other sulfides of barium are ba 2 s, bas 2 , and. The barium sulfide chemical formula is bas. In general, the chemistry of strontium is quite similar to that of calcium. Web barium and sulfur form several sulfides, of which the most studied is barium sulfide (bas) (81.07% ba),.
Solved Decide whether each pair of elements in the table
Web barium sulfide is the inorganic compound with the formula ba s. Bas is the barium compound produced on the largest scale. This is true of the most common barium. Barium sulfide is the inorganic compound with the formula bas. Web to find the formula of an ionic compound, first identify the cation and write down its symbol and charge.
It Is An Important Precursor To Other Barium.
Bas is the barium compound produced on the. Web the two ions are bound trough an ionic bond. Web the formula bas is barium sulfide.barium sulfide is an inorganic compound, and an important precursor to other barium compounds.ba = bariums =. In its compounds strontium has an exclusive oxidation state of +2, as the sr 2+ ion.
Web Write Formulas For The Binary Ionic Compounds Formed Between The Following Pairs Of Elements:
Na 2 o (sodium oxide) na 2 s (sodium sulfide) h 2 o (water) h 2 s (hydrogen sulfide) o 3 (ozone) so 2 (sulfur dioxide) co 2 (carbon. Web what is the formula of the ionic compound formed by barium and sulfur? Barium sulfide is the inorganic compound with the formula bas. Web to find the formula of an ionic compound, first identify the cation and write down its symbol and charge.
Web Barium Sulfide Is Also Known To Undergo Slow Oxidation In Solution To Form Elemental Sulfur And Various Oxidized Sulfur Species Including The Sulfite, Thiosulfate, Polythionates, And.
Web the metal is readily attacked by acids. In general, the chemistry of strontium is quite similar to that of calcium. (a) given element is barium = symbol is ( ba ) sulfur symbol is ( s ) we know that ionic compound formed from ions cation and anion cations are ( + ve ) charged. Web sulfur barium ionic compound.
Bas Is The Barium Compound Produced On The Largest Scale.
Web barium and sulfur form several sulfides, of which the most studied is barium sulfide (bas) (81.07% ba), which melts at 2200°c. Sulfuric acid is a notable exception because passivation stops the reaction by forming the insoluble barium sulfate on the surface. Other sulfides of barium are ba 2 s, bas 2 , and. Then, identify the anion and write down its symbol and charge.