Does Oxygen And Bromine Form An Ionic Compound
Does Oxygen And Bromine Form An Ionic Compound - Predict the type of compound formed from elements based on their location within the periodic table. Web would bromine and oxygen form an ionic compound? An oxygen atom gains two electrons to form an oxide ion. As a general rule, nonmetals will form covalent bonds with. Web bromine is a chemical element with the symbol br and atomic number 35. 3 can oxygen bond to bromine? Web up to 6% cash back the compound contains a metal (potassium) and a nonmetal (oxygen), so it is an ionic compound, with no transition metals. Web bromine dioxide is the chemical compound composed of bromine and oxygen with the formula bro 2. For example, cabr 2 contains a. Web in covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei.
Web up to 6% cash back the compound contains a metal (potassium) and a nonmetal (oxygen), so it is an ionic compound, with no transition metals. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Web article talk read edit view history bromine compounds are compounds containing the element bromine (br). First, compounds between metal and nonmetal elements are usually ionic. Predict the type of compound formed from elements based on their location within the periodic table. An oxygen atom gains two electrons to form an oxide ion. Web an ionic compound is made up of charged particles, called ions. Although both of these ions have. Write a formula for the ionic compound that forms between each pair of elements. Web bromine dioxide is the chemical compound composed of bromine and oxygen with the formula bro 2.
Predict the type of compound formed from elements based on their location within the periodic table. Web no it doesn't. However they from a covalent bond. Web by ibanth contents hide 1 is oxygen and bromine ionic or covalent? An oxygen atom gains two electrons to form an oxide ion. 3 can oxygen bond to bromine? Web up to 6% cash back the compound contains a metal (potassium) and a nonmetal (oxygen), so it is an ionic compound, with no transition metals. Although both of these ions have. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. An example of a covalent compound is ammonia.
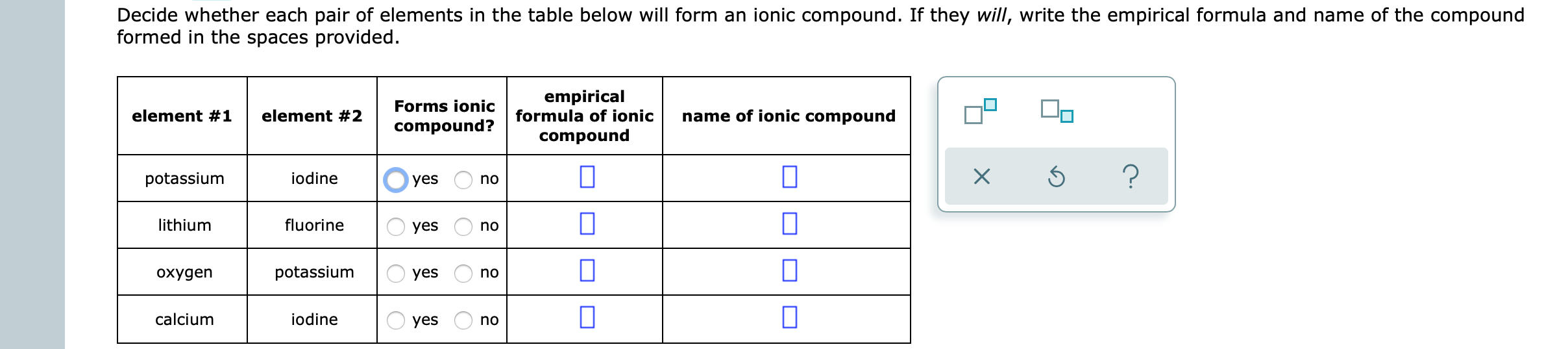
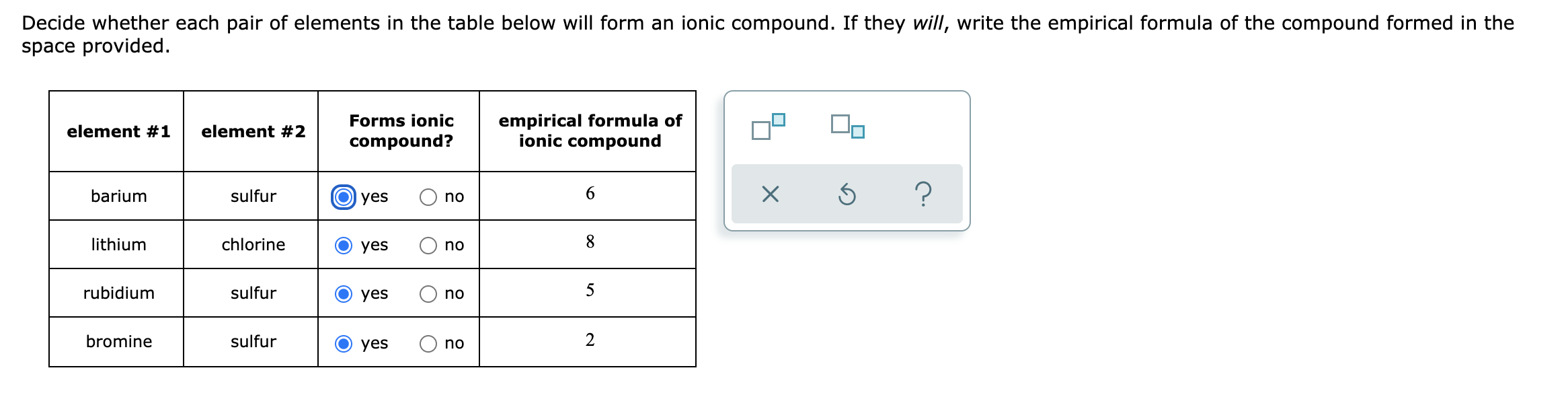
Answered Decide whether each pair of elements in… bartleby
Web bromine dioxide is the chemical compound composed of bromine and oxygen with the formula bro 2. Web bromine is a chemical element with the symbol br and atomic number 35. Dibromine monoxide (br 2 o) bromine dioxide (bro 2) dibromine trioxide (br 2 o 3) dibromine pentoxide (br. Web would bromine and oxygen form an ionic compound? Web in.
Chemistry experiment 32 Reaction between bromine and aluminium YouTube
Web bromine dioxide is the chemical compound composed of bromine and oxygen with the formula bro 2. As a general rule, nonmetals will form covalent bonds with. An example of a covalent compound is ammonia. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−.
Solved Add electron dots and charges as necessary to show
Web bromine dioxide is the chemical compound composed of bromine and oxygen with the formula bro 2. Ions are formed by the transfer of electrons. Web in covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. 2 does barium and oxygen form an ionic compound? Web no it doesn't.
Chemical Elements Bromine
Web in covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. However they from a covalent bond. Web bromine is a chemical element with the symbol br and atomic number 35. Web would bromine and oxygen form an ionic compound? Web in principle, iodine oxide (io) can also be measured by the.
Chemical Elements Bromine
Web no it doesn't. Web an ionic compound is made up of charged particles, called ions. Web in covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. As a general rule, nonmetals will form covalent bonds with. First, compounds between metal and nonmetal elements are usually ionic.
Answered Decide whether each pair of elements in… bartleby
Web an ionic compound is made up of charged particles, called ions. To name an ionic compound, the. Web bromine dioxide is the chemical compound composed of bromine and oxygen with the formula bro 2. As a general rule, nonmetals will form covalent bonds with. Web by ibanth contents hide 1 is oxygen and bromine ionic or covalent?
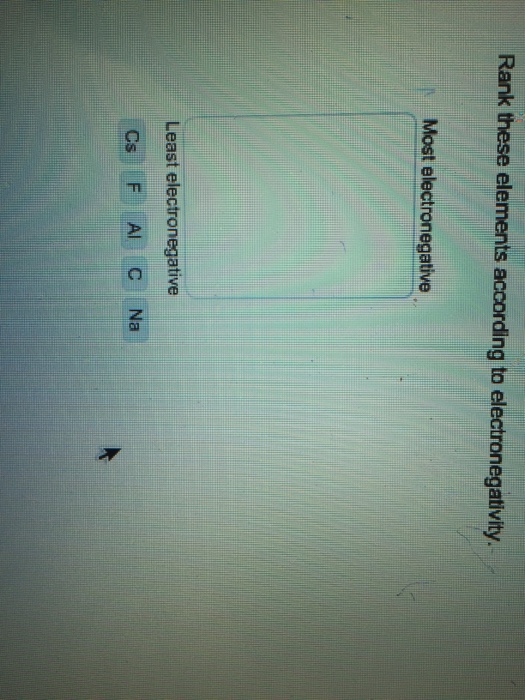
6. The most common ion of bromine has a charge of A. +2 В. +1 C
3 can oxygen bond to bromine? Web bromine is a chemical element with the symbol br and atomic number 35. An oxygen atom gains two electrons to form an oxide ion. To name an ionic compound, the. Dibromine monoxide (br 2 o) bromine dioxide (bro 2) dibromine trioxide (br 2 o 3) dibromine pentoxide (br.
science exothermic reaction sodium bromine Fundamental Photographs
Write a formula for the ionic compound that forms between each pair of elements. Web up to 6% cash back the compound contains a metal (potassium) and a nonmetal (oxygen), so it is an ionic compound, with no transition metals. Web no it doesn't. Web article talk read edit view history bromine compounds are compounds containing the element bromine (br)..
Bromine Form Periodic Table Of Elements Stock Illustration Image 7137169
Write a formula for the ionic compound that forms between each pair of elements. Web define ionic and molecular (covalent) compounds. An example of a covalent compound is ammonia. Both oxygen and bronie are nonmetals. Although both of these ions have.
Understanding Ionic Bond Formation Between Manganese And Chlorine
An oxygen atom gains two electrons to form an oxide ion. It was first isolated by. Web no it doesn't. Predict the type of compound formed from elements based on their location within the periodic table. First, compounds between metal and nonmetal elements are usually ionic.
Web Bromine Dioxide Is The Chemical Compound Composed Of Bromine And Oxygen With The Formula Bro 2.
Web an ionic compound is made up of charged particles, called ions. Web bromine is a chemical element with the symbol br and atomic number 35. To name an ionic compound, the. Dibromine monoxide (br 2 o) bromine dioxide (bro 2) dibromine trioxide (br 2 o 3) dibromine pentoxide (br.
An Oxygen Atom Gains Two Electrons To Form An Oxide Ion.
Web by ibanth contents hide 1 is oxygen and bromine ionic or covalent? It was first isolated by. However they from a covalent bond. 3 can oxygen bond to bromine?
First, Compounds Between Metal And Nonmetal Elements Are Usually Ionic.
Write a formula for the ionic compound that forms between each pair of elements. Web in principle, iodine oxide (io) can also be measured by the chemical conversion resonance fluorescence method, but this has yet to be done in the atmosphere. An example of a covalent compound is ammonia. As a general rule, nonmetals will form covalent bonds with.
For Example, Cabr 2 Contains A.
Web in covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Web up to $3 cash back 33. Web article talk read edit view history bromine compounds are compounds containing the element bromine (br). 2 does barium and oxygen form an ionic compound?