Do Noble Gases Form Ions
Do Noble Gases Form Ions - Web noble gases (group 8): Web under certain conditions, the noble gases can form diatomic gases, clathrates, fluorides, chlorides, metal complexes, and other compounds. Web the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell for elements in groups 6 and 7, the charge on the ion is equal to (8 minus group. The noble gases are very unreactive. The full valence electron shells of these atoms make. Web what charge do noble gases take as ions? For neutral atoms, the 3d orbitals are above the 4s orbitals in energy. Part of chemistry (single science) atomic. Web the atoms of noble gases already have complete outer shells, so they have no tendency to lose, gain, or share electrons. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed.
There are special names for certain types of ions. 0 (uncharged) dianions, dications, and zwitterions. Web noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. Web noble gases (group 8): Web under certain conditions, the noble gases can form diatomic gases, clathrates, fluorides, chlorides, metal complexes, and other compounds. The noble gases are very unreactive. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Web gcse wjec group 0 and testing ions flame tests identify alkali metal ions in compounds. Web the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell for elements in groups 6 and 7, the charge on the ion is equal to (8 minus group. Web noble gases are odorless, colorless, nonflammable, and monotonic gases that have low chemical reactivity.
Web the atoms of noble gases already have complete outer shells, so they have no tendency to lose, gain, or share electrons. For neutral atoms, the 3d orbitals are above the 4s orbitals in energy. This trend can be used as a guide in. Web here are five of the six noble gases: Krypton and xeon form compounds only with. Web gcse wjec group 0 and testing ions flame tests identify alkali metal ions in compounds. Group 16 elements (two groups left) form 2− ions, and so on. Web what charge do noble gases take as ions? Chemistry library > unit 7 lesson 4: Web noble gases (group 8):
Trends on the Periodic Table Chemistry Is My Jam!
Web here are five of the six noble gases: Group 16 elements (two groups left) form 2− ions, and so on. 0 (uncharged) dianions, dications, and zwitterions. Web argon chemistry atomic argon has a filled shell configuration with filled 3s and 3p orbitals. Web noble gases (group 8):
Chemistry 9/15, 9/18 Noble Gas Configurations, The Atomic Museum
Group 16 elements (two groups left) form 2− ions, and so on. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed. For neutral atoms, the 3d orbitals are above the 4s orbitals in energy. Krypton and xeon form compounds only with. Web the ions have the electronic.
Ionic Compound Nomenclature Presentation Chemistry
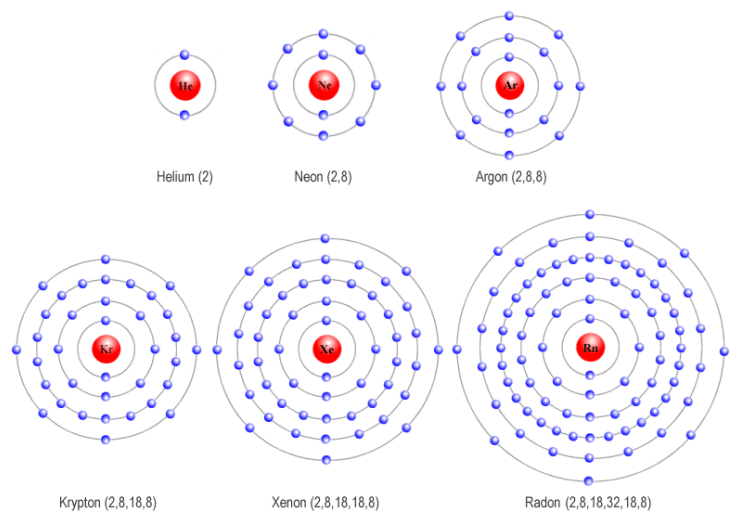
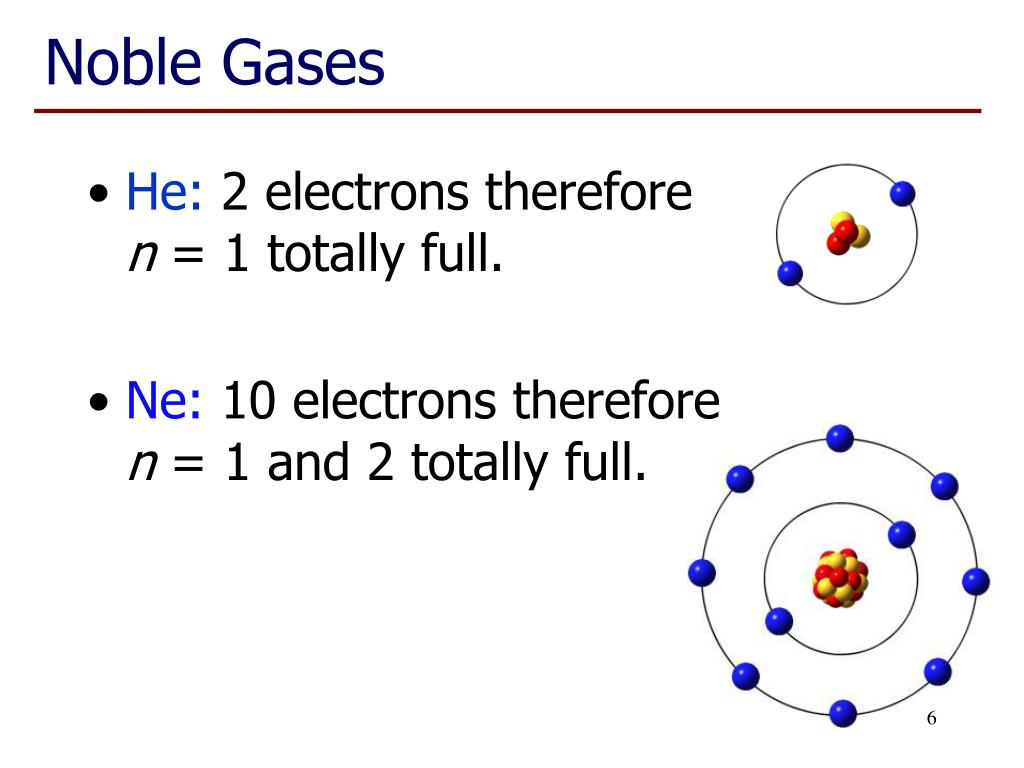
Helium, neon, argon, kypton and xeon. Group 16 elements (two groups left) form 2− ions, and so on. This is why the noble gases are inert and do not take part. 0 (uncharged) dianions, dications, and zwitterions. Web they are helium, neon, argon, krypton, xenon, and radon.
Why do Noble Gases rarely form Bonds with other Atoms? MakeTheBrainHappy
Web noble gases (group 8): This trend can be used as a guide in. Part of chemistry (single science) atomic. The noble gases of the periodic table do not have a charge because they are nonreactive. Web noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table.
Ionic Compound Nomenclature Presentation Chemistry
Web argon chemistry atomic argon has a filled shell configuration with filled 3s and 3p orbitals. Part of chemistry (single science) atomic. This is why the noble gases are inert and do not take part. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Web the ions have the electronic structure of a.
What Are the Freezing, Melting, and Boiling Points of Solids, Liquids
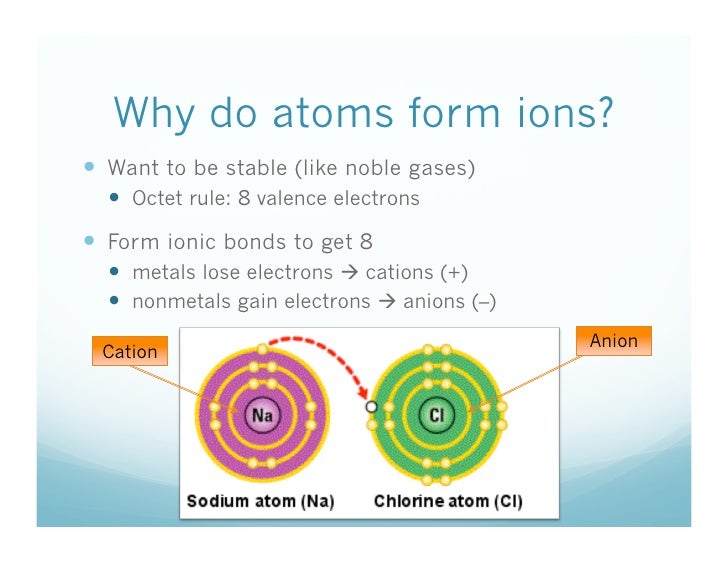
The full valence electron shells of these atoms make. Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Helium, neon, argon, kypton and xeon. Web noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table..
Chemical Bonding
Web gcse wjec group 0 and testing ions flame tests identify alkali metal ions in compounds. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; This is why the noble gases are inert and do not take part. Web ionic bonding is the complete transfer of valence electron (s) between atoms and is.
10 28 How Many Electrons Do Atoms Gain Lose
Web noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. Group 16 elements (two groups left) form 2− ions, and so on. Web what charge do noble gases take as ions? The elements are helium (he), neon (ne), argon (ar), krypton. Web the ions have the electronic structure of a noble.
PPT Recap Atomic Structure PowerPoint Presentation, free download
For neutral atoms, the 3d orbitals are above the 4s orbitals in energy. Web under certain conditions, the noble gases can form diatomic gases, clathrates, fluorides, chlorides, metal complexes, and other compounds. Web noble gases are odorless, colorless, nonflammable, and monotonic gases that have low chemical reactivity. The full valence electron shells of these atoms make. Web here are five.
What Are Noble Gases? Definition and Properties
Web gcse wjec group 0 and testing ions flame tests identify alkali metal ions in compounds. The noble gases of the periodic table do not have a charge because they are nonreactive. Electron configurations shells, subshells, and orbitals introduction to electron configurations noble gas. The full valence electron shells of these atoms make. This trend can be used as a.
Web Under Certain Conditions, The Noble Gases Can Form Diatomic Gases, Clathrates, Fluorides, Chlorides, Metal Complexes, And Other Compounds.
The full valence electron shells of these atoms make. Web the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell for elements in groups 6 and 7, the charge on the ion is equal to (8 minus group. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. Group 16 elements (two groups left) form 2− ions, and so on.
Electron Configurations Shells, Subshells, And Orbitals Introduction To Electron Configurations Noble Gas.
Web they are helium, neon, argon, krypton, xenon, and radon. Web here are five of the six noble gases: Web noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. Web noble gases (group 8):
A Noble Gas Configuration Of An Atom Consists Of The Elemental Symbol Of The Last Noble Gas Prior To That Atom, Followed.
For neutral atoms, the 3d orbitals are above the 4s orbitals in energy. Helium, neon, argon, kypton and xeon. Krypton and xeon form compounds only with. Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged ions.
There Are Special Names For Certain Types Of Ions.
They're all colourless and transparent. Web gcse wjec group 0 and testing ions flame tests identify alkali metal ions in compounds. Chemistry library > unit 7 lesson 4: 0 (uncharged) dianions, dications, and zwitterions.


.PNG)
.PNG)