Chemistry Chapter 2 Review Answer Key
Chemistry Chapter 2 Review Answer Key - A molecule of oxygen, o 2, contains two oxygen atoms; Web chapter 2 chemistry review. Report the number of significant figures in each of the following values:. 17.5 batteries and fuel cells; The chemistry of life review questions. E) 7055 mm (3) 7 x 10 3 mm f. 17.3 electrode and cell potentials; Web chemistry chapter 2 test review quiz for 10th grade students. The starting materials consist of one green sphere and two purple spheres. Web the electron pulled into the nucleus was most likely found in the 1 s orbital.
Web terms in this set (44) what is the fundamental building block of matter? Factors that accelerate the rate of chemical. Web b) a decomposition reaction. Any substance that is made up of one atom is called a (n) _______. C) (166 ps) + (3 ps) d) (15 km) (5 h) answer: Web chemistry unit 2 review guide 1. An ________ is a property that depends on the type of matter in a sample. List some differences between a script and a function. A) 142830 km (3) 1 x 10 5 km b) 79 ms (2) 8 x10 1 ms 143000 c) 0 g (4) 0 ug d) 89 mg (3) 90 mg; Matter that has a uniform and definite composition is called a.
A molecule of oxygen, o 2, contains two oxygen atoms; Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. Factors that accelerate the rate of chemical. Web sci 10 chapter 2 review questions.doc — microsoft word document, 61 kb (62976 bytes) An ________ is a property that depends on the type of matter in a sample. Web 5.0 (3 reviews) how does quantitative information differ from qualitative information? Web chapter 2 review measurements and calculations section 3 short answer answer the following questions in the space provided. ____ and _____ are examples of pure. This violates dalton’s postulate that that. The products consist of two green spheres and two purple spheres.
Chapter 2 Review Answers
(2) c) an exchange reaction. And the mass number is 12. E) 7055 mm (3) 7 x 10 3 mm f. Any substance that is made up of one atom is called a (n) _______. Web 5.0 (3 reviews) how does quantitative information differ from qualitative information?
modern chemistry chapter 2 test
(1) a) a synthesis reaction. Web chemistry unit 2 review guide 1. 17.3 electrode and cell potentials; (2) hcl + naoh → nacl + h2o. Click the card to flip 👆.
18 Best Images of Biology Worksheet Answer Key Chapter 23 Biology
E) 7055 mm (3) 7 x 10 3 mm f. Web 7468 chapter 2 review answer key chemistry | updated 2174 kb/s 9997 chapter 2 review answer key chemistry | new 4288 kb/s 1808 chapter 2 review answer key chemistry [most popular] 3517 kb/s 6660 how to get a new electronic car key replacing an electronic key. 17.3 electrode and.
Prentice Hall Brief Review Chemistry 2021 Answer Key TOP
A molecule of oxygen, o 2, contains two oxygen atoms; (2) hcl + naoh → nacl + h2o. C) (166 ps) + (3 ps) d) (15 km) (5 h) answer: (1) a) a synthesis reaction. What is the difference between naming an ionic compound and a binary molecular compound?
20 Best Images of Molecules Worksheet Answers Key Of Life Building
Web chemistry unit 2 review guide 1. Our resource for chemistry includes answers to chapter exercises, as well as detailed. Web 5.0 (3 reviews) how does quantitative information differ from qualitative information? Simple ionic compounds are named by: 17.3 electrode and cell potentials;
Honors Chemistry Final Exam Answer Key General Chemistry Cheat Sheet
The starting materials consist of one green sphere and two purple spheres. Our resource for chemistry includes answers to chapter exercises, as well as detailed. What is the difference between naming an ionic compound and a binary molecular compound? As an electron falls from a higher energy level to replace it, the difference in the energy of the replacement electron.
16 Best Images of Chemistry Naming Compounds Worksheet Answers
Web terms in this set (44) what is the fundamental building block of matter? (2) c) an exchange reaction. 17.5 batteries and fuel cells; This violates dalton’s postulate that that. Our resource for chemistry includes answers to chapter exercises, as well as detailed.
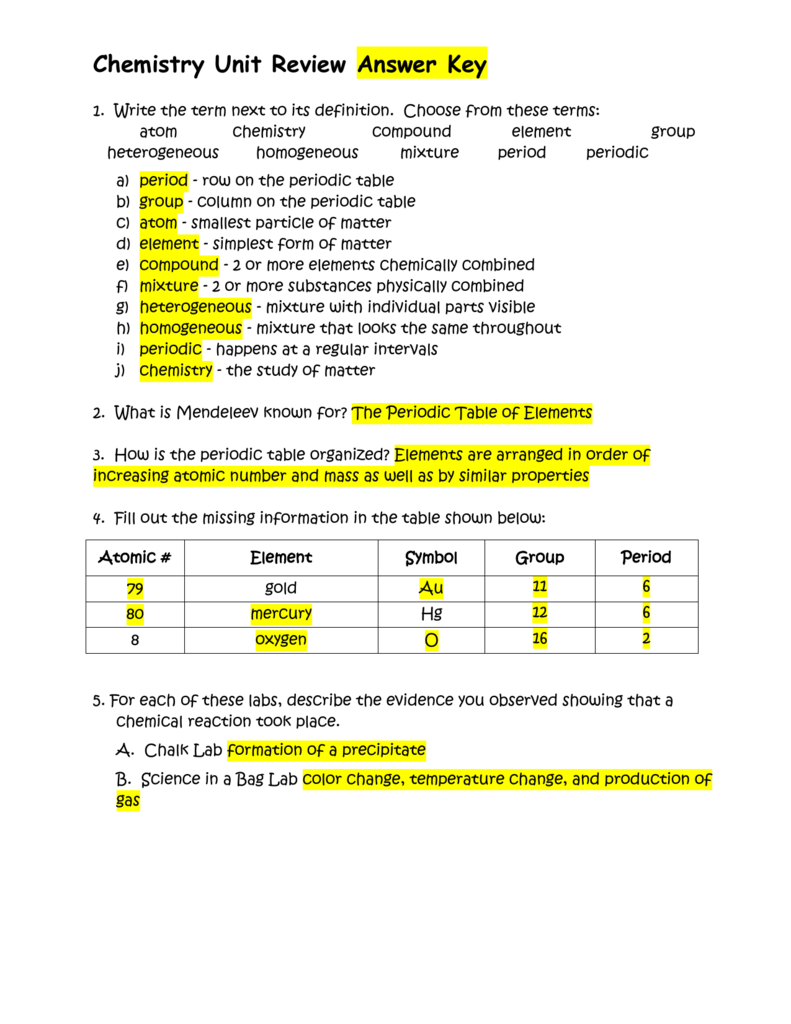
Chemistry Unit Review Answer Key
The subscript 2 in the formula must be used to distinguish the. C) (166 ps) + (3 ps) d) (15 km) (5 h) answer: And the mass number is 12. Web terms in this set (44) what is the fundamental building block of matter? Any substance that is made up of one atom is called a (n) _______.
Chemical Equilibrium Worksheet Answers. Worksheets. Tutsstar Thousands
The products consist of two green spheres and two purple spheres. Report the number of significant figures in each of the following values:. 17.5 batteries and fuel cells; Web chapter 2 chemistry review. Web 5.0 (3 reviews) how does quantitative information differ from qualitative information?
Both Types Of Bonds Result From Overlap Of Atomic Orbitals On Adjacent Atoms And Contain A Maximum Of Two Electrons.
A) 142830 km (3) 1 x 10 5 km b) 79 ms (2) 8 x10 1 ms 143000 c) 0 g (4) 0 ug d) 89 mg (3) 90 mg; (2) c) an exchange reaction. The chemistry of life review questions. 17.3 electrode and cell potentials;
Web 17.1 Review Of Redox Chemistry;
E) 7055 mm (3) 7 x 10 3 mm f. Web terms in this set (44) what is the fundamental building block of matter? Web chemistry chapter 2 test review quiz for 10th grade students. The products consist of two green spheres and two purple spheres.
Find Other Quizzes For Chemistry And More On Quizizz For Free!
And the mass number is 12. (1) 2hg + o2 → s 2hgo. ____ and _____ are examples of pure. Factors that accelerate the rate of chemical.
Web 5.0 (3 Reviews) How Does Quantitative Information Differ From Qualitative Information?
Report the number of significant figures in each of the following values:. Web chapter 2 review measurements and calculations section 3 short answer answer the following questions in the space provided. This violates dalton’s postulate that that. As an electron falls from a higher energy level to replace it, the difference in the energy of the replacement electron in its two energy.