Chapter 7 Chemistry Test
Chapter 7 Chemistry Test - Negative ions negative ions are called? Valence electrons what is formed when atoms gain electrons? Click the card to flip 👆 1 / 11 flashcards learn test. 7.6 molecular structure and polarity; Web 7.5 strengths of ionic and covalent bonds; N2h4(l) + h2(g) → 2nh3(g) reaction 2: Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? 48 questions | by konlee | updated: Mar 21, 2022 | attempts: Web octet metals are reactive because they lose what easily?
N2h4(l) + h2(g) → 2nh3(g) reaction 2: Negative ions negative ions are called? Click the card to flip 👆 1 / 11 flashcards learn test. Web 7.5 strengths of ionic and covalent bonds; Web chapter 7 chem: Web octet metals are reactive because they lose what easily? 48 questions | by konlee | updated: Mar 21, 2022 | attempts: Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Valence electrons what is formed when atoms gain electrons?
Valence electrons what is formed when atoms gain electrons? N2h4(l) + h2(g) → 2nh3(g) reaction 2: Click the card to flip 👆 1 / 11 flashcards learn test. Web octet metals are reactive because they lose what easily? 7.6 molecular structure and polarity; 48 questions | by konlee | updated: Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Mar 21, 2022 | attempts: Negative ions negative ions are called? Web 7.5 strengths of ionic and covalent bonds;
Chapter 7 Chemistry Test Answers Study Finder
Mar 21, 2022 | attempts: Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Web 7.5 strengths of ionic and covalent bonds; Negative ions negative ions are called? Click the card to flip 👆 1 / 11 flashcards learn test.
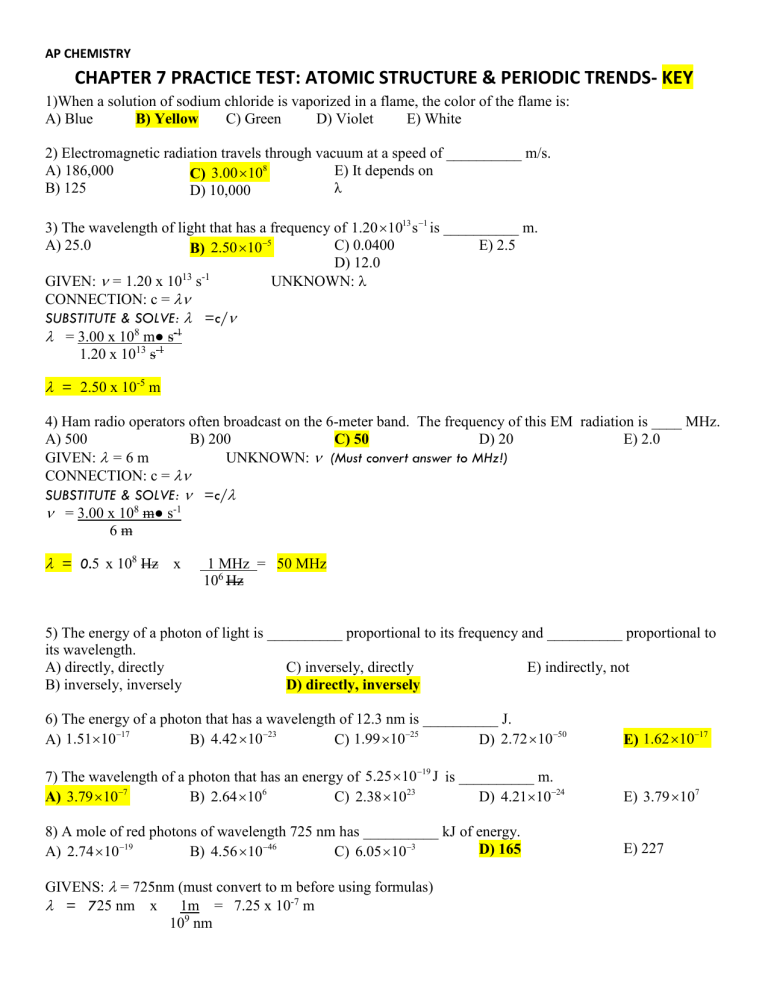
AP CHEMISTRY CHAPTER 7 PRACTICE TEST ATOMIC
Web 7.5 strengths of ionic and covalent bonds; Web chapter 7 chem: Mar 21, 2022 | attempts: Negative ions negative ions are called? N2h4(l) + h2(g) → 2nh3(g) reaction 2:
modern chemistry chapter 1 test
N2h4(l) + h2(g) → 2nh3(g) reaction 2: 7.6 molecular structure and polarity; Mar 21, 2022 | attempts: 48 questions | by konlee | updated: Valence electrons what is formed when atoms gain electrons?
NCERT Exemplar Class 12 Chemistry Solutions Chapter 7 The P Block
Valence electrons what is formed when atoms gain electrons? Negative ions negative ions are called? Web octet metals are reactive because they lose what easily? Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Click the card to flip 👆 1 / 11.
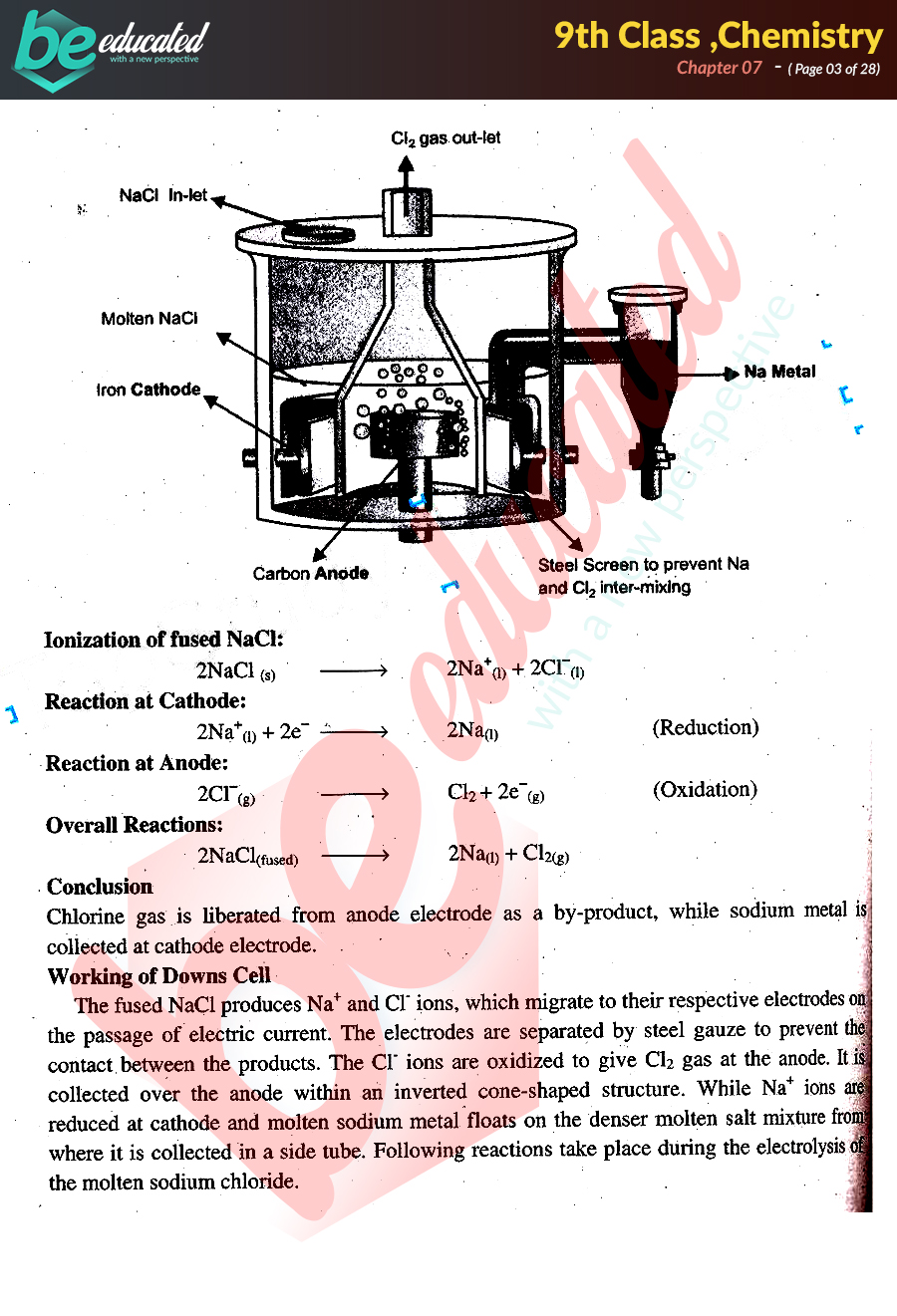
Chapter 7 Chemistry 9th Class Notes Matric Part 1 Notes
7.6 molecular structure and polarity; Web 7.5 strengths of ionic and covalent bonds; Web chapter 7 chem: 48 questions | by konlee | updated: Mar 21, 2022 | attempts:
Chemistry Chapter 7 Worksheet Answers —
Negative ions negative ions are called? Web octet metals are reactive because they lose what easily? Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? N2h4(l) + h2(g) → 2nh3(g) reaction 2: 7.6 molecular structure and polarity;
Chemistry Chapter 7 2nd Year
Mar 21, 2022 | attempts: Web chapter 7 chem: 48 questions | by konlee | updated: Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? N2h4(l) + h2(g) → 2nh3(g) reaction 2:
Chapter 7 Chemistry YouTube
7.6 molecular structure and polarity; Mar 21, 2022 | attempts: Click the card to flip 👆 1 / 11 flashcards learn test. Web octet metals are reactive because they lose what easily? Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end?
Chapter 7 Test Review
Web 7.5 strengths of ionic and covalent bonds; Negative ions negative ions are called? Web octet metals are reactive because they lose what easily? Valence electrons what is formed when atoms gain electrons? Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end?
S block elements Chapter 7 Chemistry Class 11 MHTCET 2020 2021
N2h4(l) + h2(g) → 2nh3(g) reaction 2: 48 questions | by konlee | updated: Web octet metals are reactive because they lose what easily? Web 7.5 strengths of ionic and covalent bonds; Valence electrons what is formed when atoms gain electrons?
7.6 Molecular Structure And Polarity;
Web octet metals are reactive because they lose what easily? Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Negative ions negative ions are called? Click the card to flip 👆 1 / 11 flashcards learn test.
N2H4(L) + H2(G) → 2Nh3(G) Reaction 2:
Web chapter 7 chem: Mar 21, 2022 | attempts: Web 7.5 strengths of ionic and covalent bonds; 48 questions | by konlee | updated: