Chapter 4 Chemistry Test Answer Key
Chapter 4 Chemistry Test Answer Key - Ap chemistry chapter practice exam the claus reactions, shown below, are used to (b) 3 cu ( s) + 8 hno 3 ( a q) 3 cu ( no 3) 2 ( a q) + 4. Our resource for holt chemistry includes answers to chapter exercises, as well as. Know how to calculate number of electrons per energy level. $7.22 ) (no reviews yet) write a. Equations must be balanced to. A) nano 3 c) o 2 d) oil (c xh y) e) sugar (c 11 h 22 o 12) h) wax (c xh. Its practically what you dependence currently. Please check your connection and try again. Know the definition of electron cloud, the quantum numbers, orbital.
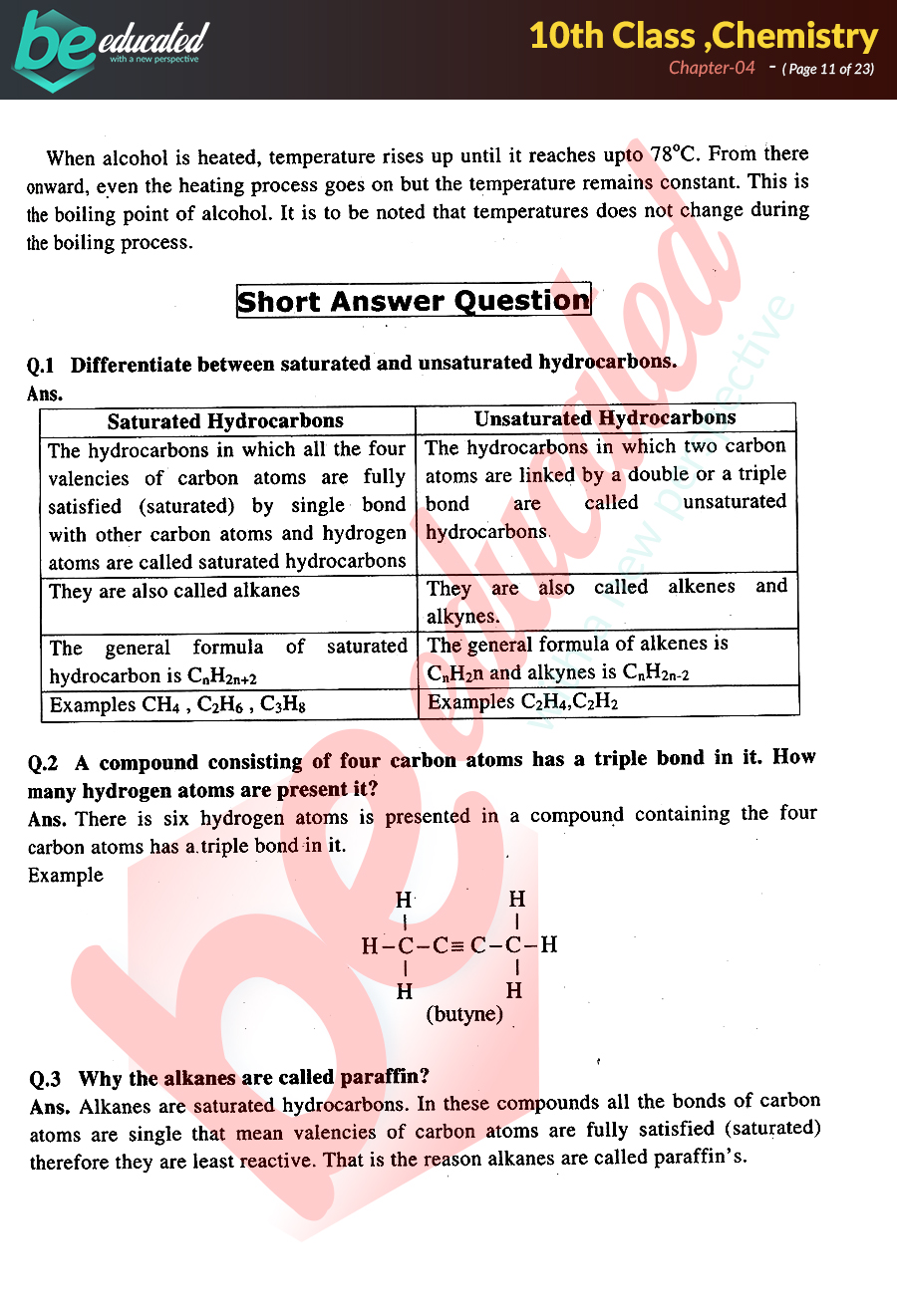
This chapter 4 chemistry test answers , as one of the most lively sellers here will extremely be in the middle of the best options to review. Web 11th grade chemistry chapter 4 test quiz for 11th grade students. An equation is balanced when the same number of each element is represented on the reactant and product sides. 0, 1, 2, 3 d. An equation is balanced when the same. All of the elements exist in approximately equal amounts. Web ap chemistry test (chapter 4) please use the answer sheet!! Atoms of different elements are different 3. Select one or more questions using the checkboxes above each question. An equation is balanced when the same number of each element is represented on the reactant and product sides.
We're unable to load study guides on this page. Equations must be balanced to accurately reflect the law of conservation of matter. All of the elements exist in approximately equal amounts. Web study with quizlet and memorize flashcards containing terms like match the terms to their correct answers _____ 1. All matter is composed of extremely small particles called atoms 2. Web chapter 4 practice test key provided by dr. The principal quantum number of an electron is 4. Then click the add selected questions to a test. Ap chemistry chapter practice exam the claus reactions, shown below, are used to What are the possible angular momentum quantum numbers?
Chapter 4 Assessment Chemistry Answer Key 38+ Pages Answer Doc [1.2mb
What is the total number of electrons needed to fill the fourth main energy level? What are the possible angular momentum quantum numbers? Web ap chemistry test (chapter 4) please use the answer sheet!! Find other quizzes for chemistry and more on quizizz for free! An equation is balanced when the same number of each element is represented on the.
Chemistry Chapter 4 Notes Day 1 YouTube
Web find on the ap chemistry exam and the sat chemistry subject test high school chemistry unlocked covers: Web 11th grade chemistry chapter 4 test quiz for 11th grade students. Please check your connection and try again. This test answer key for chemistry provides a copy of the tests with the correct answers overlaid. Web chemistry chapter 4 arrangement of.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 4 Chemical
Know the definition of electron cloud, the quantum numbers, orbital. Web fourth grade (grade 4) chemistry questions. Please check your connection and try again. Equations must be balanced to accurately reflect the law of conservation of matter. Web chapter 4 practice test key provided by dr.
Chapter 4 Chemistry 10th Class Notes Matric Part 2 Notes
Web find on the ap chemistry exam and the sat chemistry subject test high school chemistry unlocked covers: An equation is balanced when the same. Select one or more questions using the checkboxes above each question. Web fourth grade (grade 4) chemistry questions. This chapter 4 chemistry test answers , as one of the most lively sellers here will extremely.
Chapter 4 Chemistry 10th Class Notes Matric Part 2 Notes
Web quantum the amount of energy required to move an electron from one energy level to the next quantum leap when an electron moves up or down electron levels photon a particle of electromagnetic radiation. Web 11th grade chemistry chapter 4 test quiz for 11th grade students. Web ap chemistry test (chapter 4) please use the answer sheet!! Know the.
Chapter 4 Chemistry 10th Class Notes Matric Part 2 Notes
Web answers and explanations for test 1. Then click the add selected questions to a test. Web chemistry chapter 4 arrangement of electrons in atoms. Its practically what you dependence currently. On the actual sat subject test in chemistry, this type of question must be answered in a special section of your answer answer key.
Unit Four Review H Key
Web answers and explanations for test 1. Web fourth grade (grade 4) chemistry questions. Web chapter 4 practice test key provided by dr. 3, 2, 1, 0, 1, 2, 3 c. An equation is balanced when the same.
Answer key for test review.
Web find on the ap chemistry exam and the sat chemistry subject test high school chemistry unlocked covers: (b) 3 cu ( s) + 8 hno 3 ( a q) 3 cu ( no 3) 2 ( a q) + 4. Web 11th grade chemistry chapter 4 test quiz for 11th grade students. Find other quizzes for chemistry and more.
Chapter 4 Chemistry 9th Class Notes Matric Part 1 Notes
Web study with quizlet and memorize flashcards containing terms like match the terms to their correct answers _____ 1. This chapter 4 chemistry test answers , as one of the most lively sellers here will extremely be in the middle of the best options to review. On the actual sat subject test in chemistry, this type of question must be.
Spice of Lyfe Chemical Equations And Reactions Chapter 8 Test
Web fourth grade (grade 4) chemistry questions. Ap chemistry chapter practice exam the claus reactions, shown below, are used to Web ap chemistry test (chapter 4) please use the answer sheet!! Then click the add selected questions to a test. Web chemistry chapter 4 arrangement of electrons in atoms.
Know How To Calculate Number Of Electrons Per Energy Level.
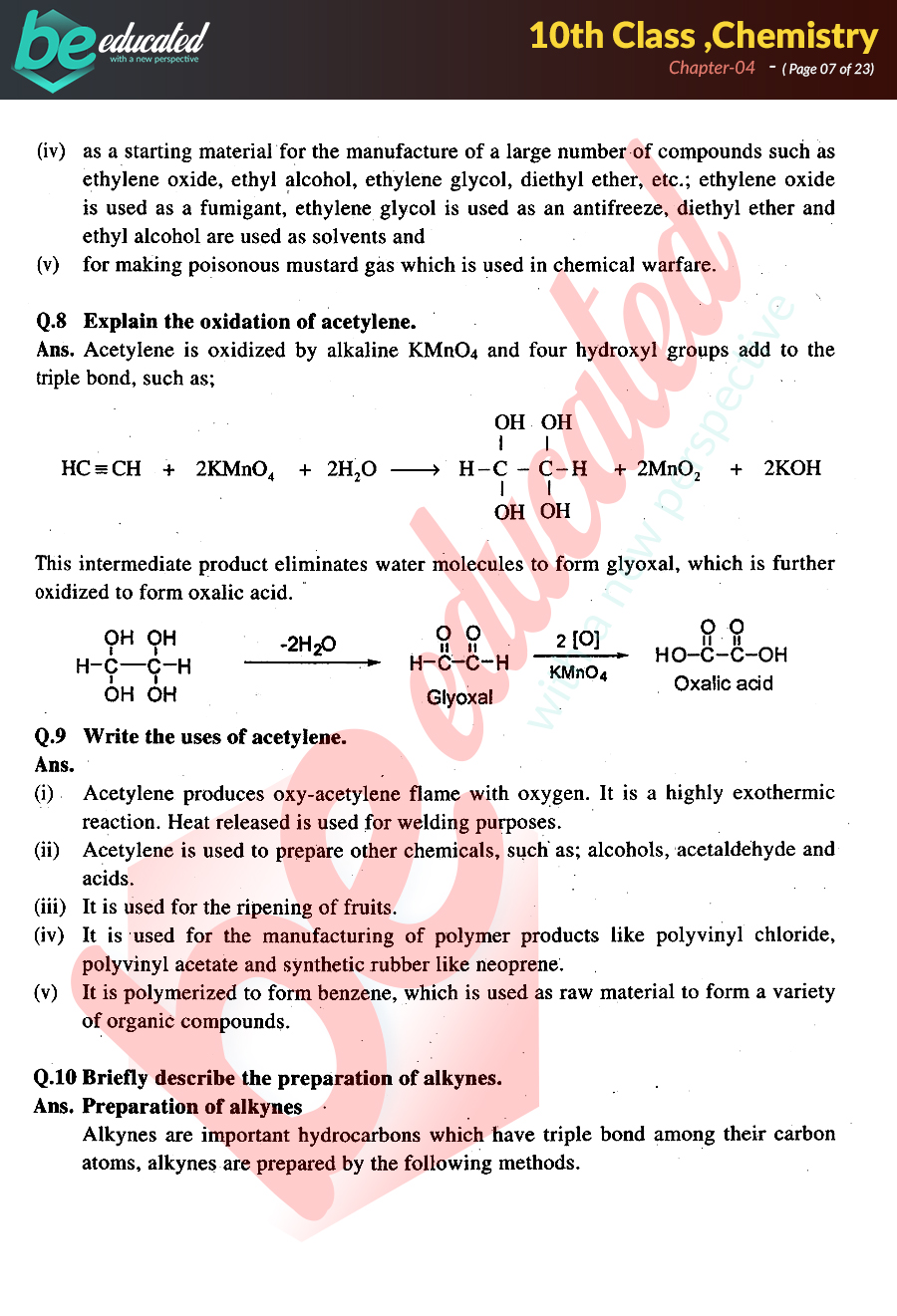
(a) pcl 5 ( s) + h 2 o ( l) pocl 3 ( l) + 2 hcl ( a q ); Equations must be balanced to. $7.22 ) (no reviews yet) write a. Web ap chemistry test (chapter 4) please use the answer sheet!!
Quiz For Jigsaw—Properties Of Liquids 1.
You can create printable tests and worksheets from these grade 4 chemistry questions! Find other quizzes for chemistry and more on quizizz for free! • building blocks of matter • physical behavior of matter • chemical bonding • chemical reactions • stoichiometry • solutions • acids and bases • equilibrium • organic chemistry. Web find on the ap chemistry exam and the sat chemistry subject test high school chemistry unlocked covers:
This Test Answer Key For Chemistry Provides A Copy Of The Tests With The Correct Answers Overlaid.
All elements are found in their uncombined form in nature. On the actual sat subject test in chemistry, this type of question must be answered in a special section of your answer answer key. 3, 2, 1, 0, 1, 2, 3 c. Web chapter 4 practice test key provided by dr.
An Equation Is Balanced When The Same Number Of Each Element Is Represented On The Reactant And Product Sides.
Web quantum the amount of energy required to move an electron from one energy level to the next quantum leap when an electron moves up or down electron levels photon a particle of electromagnetic radiation. Its practically what you dependence currently. Web answers and explanations for test 1. Web 11th grade chemistry chapter 4 test quiz for 11th grade students.