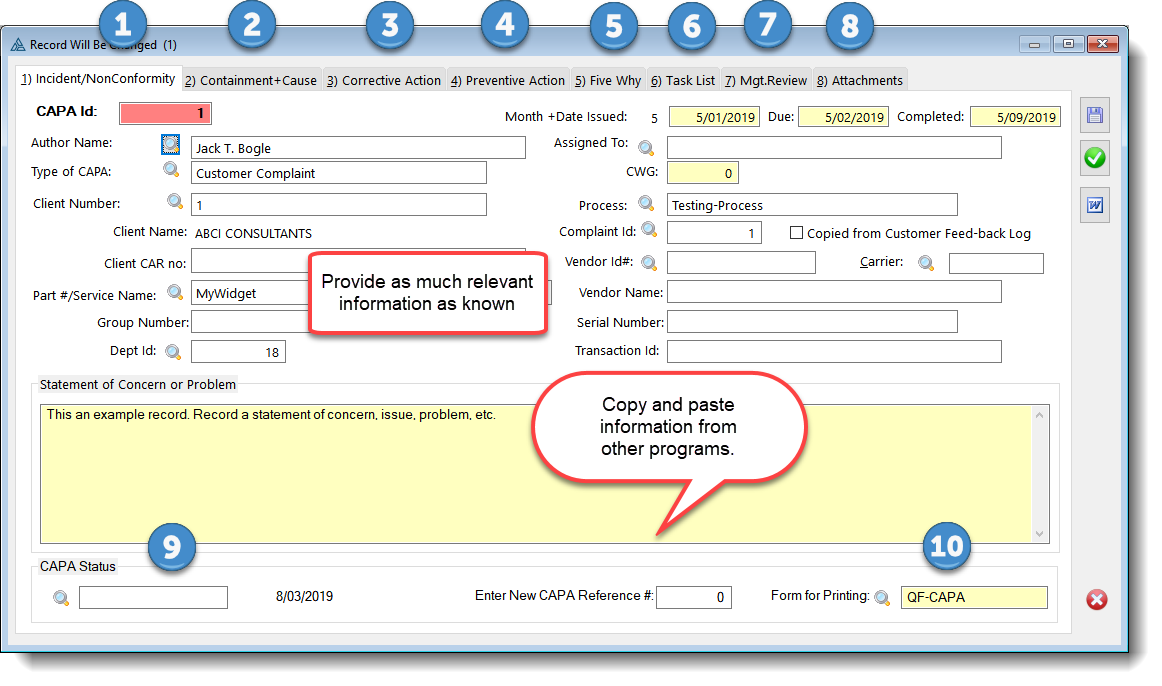
Capa Full Form
Capa Full Form - Download capa format / template. Serial number, commencing at 001 for each department in the calendar year. Department head shall decide the need for capa with head qa. The initiation of capa requires submission of the source document by concerned department head to qa. Verify that capa system procedure(s) that address the requirements of the quality system regulation have been defined and. When something goes wrong, a capa is executed to fix ('correct') the immediate issue, then to ' prevent ' it reoccurring with some tweak or improvement that addresses the cause. The american board of perianesthesia nursing certification, inc. Web corrective action preventive action (capa) is a process which investigates and solves problems, identifies causes, takes corrective action and prevents recurrence of the root causes. The department head shall get a capa form issued from qa. Qa shall write the source document name and source document number on the form before issuing of.
When something goes wrong, a capa is executed to fix ('correct') the immediate issue, then to ' prevent ' it reoccurring with some tweak or improvement that addresses the cause. The american board of perianesthesia nursing certification, inc. Free download qms & ehs template/format. Web corrective action preventive action (capa) is a process which investigates and solves problems, identifies causes, takes corrective action and prevents recurrence of the root causes. Verify that capa system procedure(s) that address the requirements of the quality system regulation have been defined and. (abpanc) is responsible for developing, sponsoring and managing the certified post anesthesia nurse (cpan ®) and the certified ambulatory perianesthesia nurse (capa ®) nursing certification programs.these national professional certification. Qa shall write the source document name and source document number on the form before issuing of. Serial number, commencing at 001 for each department in the calendar year. Last two digits of a calendar year. Web background capa (corrective and preventive action) is rooted in creating quality management systems and the requirement for ongoing growth within organizations.
The department head shall get a capa form issued from qa. The american board of perianesthesia nursing certification, inc. It is usually a set of actions, laws or regulations required by an organization to take in manufacturing, documentation, procedures, or systems. Qa shall write the source document name and source document number on the form before issuing of. The ultimate purpose of capa is to assure the. Free download qms & ehs template/format. (abpanc) is responsible for developing, sponsoring and managing the certified post anesthesia nurse (cpan ®) and the certified ambulatory perianesthesia nurse (capa ®) nursing certification programs.these national professional certification. Verify that capa system procedure(s) that address the requirements of the quality system regulation have been defined and. Web capa stands for 'corrective and preventive action'. Web corrective and preventive action (capa) is a concept with current good manufacturing practice (cgmp) that focuses on the systematic investigation of root causes of unexpected incidences to prevent their recurrence (corrective action) or to prevent their occurrence (preventive action) handling of corrective and preventive action (capa) 1.0.
Capa Form Template Free Printable Templates
(abpanc) is responsible for developing, sponsoring and managing the certified post anesthesia nurse (cpan ®) and the certified ambulatory perianesthesia nurse (capa ®) nursing certification programs.these national professional certification. Last two digits of a calendar year. Download capa format / template. The american board of perianesthesia nursing certification, inc. Serial number, commencing at 001 for each department in the calendar.
Sample Capa form Best Of 8d Report On Capa Item Report template
Web background capa (corrective and preventive action) is rooted in creating quality management systems and the requirement for ongoing growth within organizations. Web capa stands for 'corrective and preventive action'. Last two digits of a calendar year. Web corrective and preventive actions (capa) inspectional objectives. The department head shall get a capa form issued from qa.
CAPA Performance
Web cpan ® and capa ® certification. Serial number, commencing at 001 for each department in the calendar year. Web corrective and preventive actions (capa) inspectional objectives. Last two digits of a calendar year. When something goes wrong, a capa is executed to fix ('correct') the immediate issue, then to ' prevent ' it reoccurring with some tweak or improvement.
Sample Capa form Peterainsworth
Serial number, commencing at 001 for each department in the calendar year. Web corrective and preventive actions (capa) inspectional objectives. Free download qms & ehs template/format. When something goes wrong, a capa is executed to fix ('correct') the immediate issue, then to ' prevent ' it reoccurring with some tweak or improvement that addresses the cause. Web corrective action preventive.
CAPA Performance
Web capa stands for 'corrective and preventive action'. Free download qms & ehs template/format. Web a typical capa form shall be numbered as capa/xxx/yyy/z where, xxx: Department head shall decide the need for capa with head qa. The initiation of capa requires submission of the source document by concerned department head to qa.
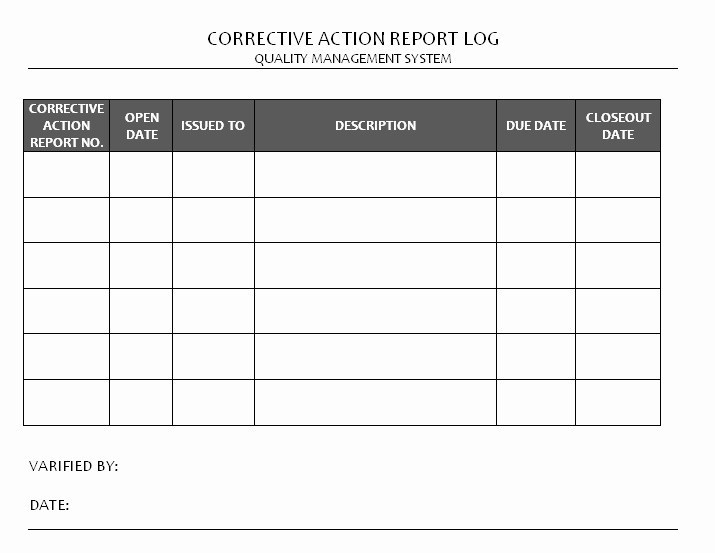
Corrective Action & Preventive Action Form
Web capa stands for 'corrective and preventive action'. Last two digits of a calendar year. The ultimate purpose of capa is to assure the. Web cpan ® and capa ® certification. It is usually a set of actions, laws or regulations required by an organization to take in manufacturing, documentation, procedures, or systems.
CAPA Form.xls Computing Technology
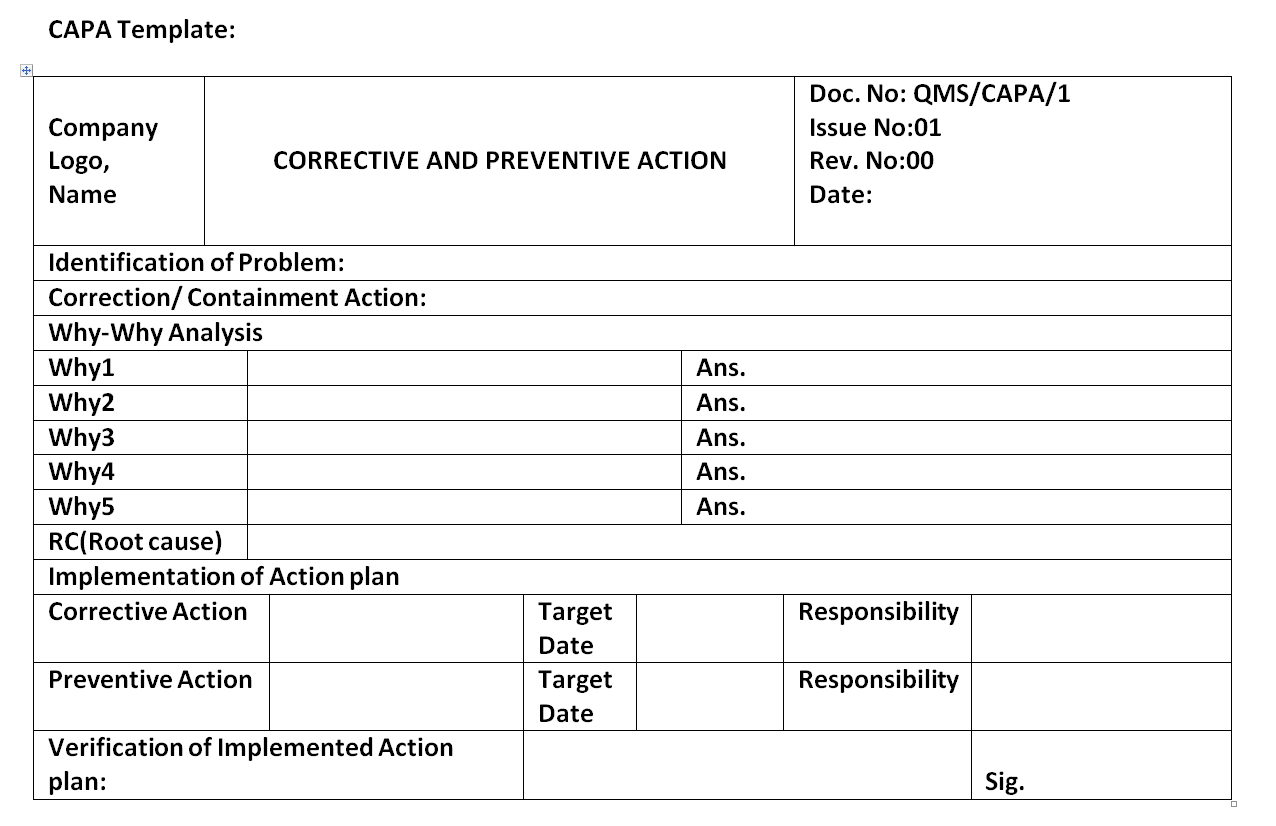
When something goes wrong, a capa is executed to fix ('correct') the immediate issue, then to ' prevent ' it reoccurring with some tweak or improvement that addresses the cause. Verify that capa system procedure(s) that address the requirements of the quality system regulation have been defined and. Capa/prd/007/15 represents the 7th capa from production department in the calendar year.
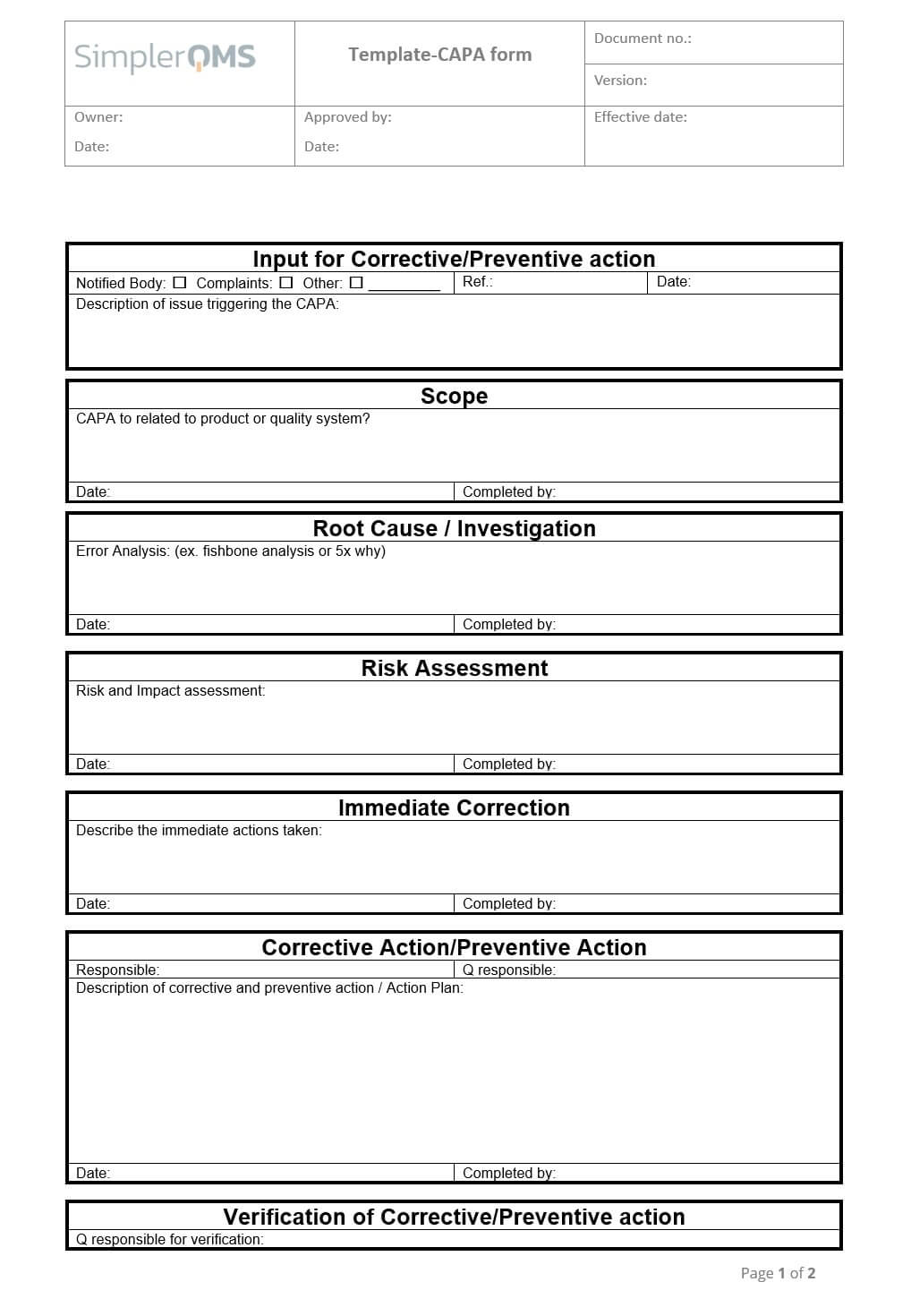
Corrective and Preventive Action (CAPA) Form Template SimplerQMS
The american board of perianesthesia nursing certification, inc. Web a typical capa form shall be numbered as capa/xxx/yyy/z where, xxx: It is usually a set of actions, laws or regulations required by an organization to take in manufacturing, documentation, procedures, or systems. Web corrective and preventive actions (capa) inspectional objectives. The ultimate purpose of capa is to assure the.
Corrective and Preventive Action Format CAPA with Example Download
(abpanc) is responsible for developing, sponsoring and managing the certified post anesthesia nurse (cpan ®) and the certified ambulatory perianesthesia nurse (capa ®) nursing certification programs.these national professional certification. Qa shall write the source document name and source document number on the form before issuing of. Department head shall decide the need for capa with head qa. The american board.
Sample Capa form Peterainsworth
The department head shall get a capa form issued from qa. Capa/prd/007/15 represents the 7th capa from production department in the calendar year 2015 Web cpan ® and capa ® certification. Serial number, commencing at 001 for each department in the calendar year. It is usually a set of actions, laws or regulations required by an organization to take in.
Web Cpan ® And Capa ® Certification.
When something goes wrong, a capa is executed to fix ('correct') the immediate issue, then to ' prevent ' it reoccurring with some tweak or improvement that addresses the cause. The department head shall get a capa form issued from qa. The american board of perianesthesia nursing certification, inc. Download capa format / template.
Verify That Capa System Procedure(S) That Address The Requirements Of The Quality System Regulation Have Been Defined And.
The ultimate purpose of capa is to assure the. The initiation of capa requires submission of the source document by concerned department head to qa. Serial number, commencing at 001 for each department in the calendar year. Web corrective action preventive action (capa) is a process which investigates and solves problems, identifies causes, takes corrective action and prevents recurrence of the root causes.
It Is Usually A Set Of Actions, Laws Or Regulations Required By An Organization To Take In Manufacturing, Documentation, Procedures, Or Systems.
Department head shall decide the need for capa with head qa. Capa/prd/007/15 represents the 7th capa from production department in the calendar year 2015 Web corrective and preventive actions (capa) inspectional objectives. Qa shall write the source document name and source document number on the form before issuing of.
Web A Typical Capa Form Shall Be Numbered As Capa/Xxx/Yyy/Z Where, Xxx:
(abpanc) is responsible for developing, sponsoring and managing the certified post anesthesia nurse (cpan ®) and the certified ambulatory perianesthesia nurse (capa ®) nursing certification programs.these national professional certification. Web corrective and preventive action (capa) is a concept with current good manufacturing practice (cgmp) that focuses on the systematic investigation of root causes of unexpected incidences to prevent their recurrence (corrective action) or to prevent their occurrence (preventive action) handling of corrective and preventive action (capa) 1.0. Web capa stands for 'corrective and preventive action'. Web background capa (corrective and preventive action) is rooted in creating quality management systems and the requirement for ongoing growth within organizations.