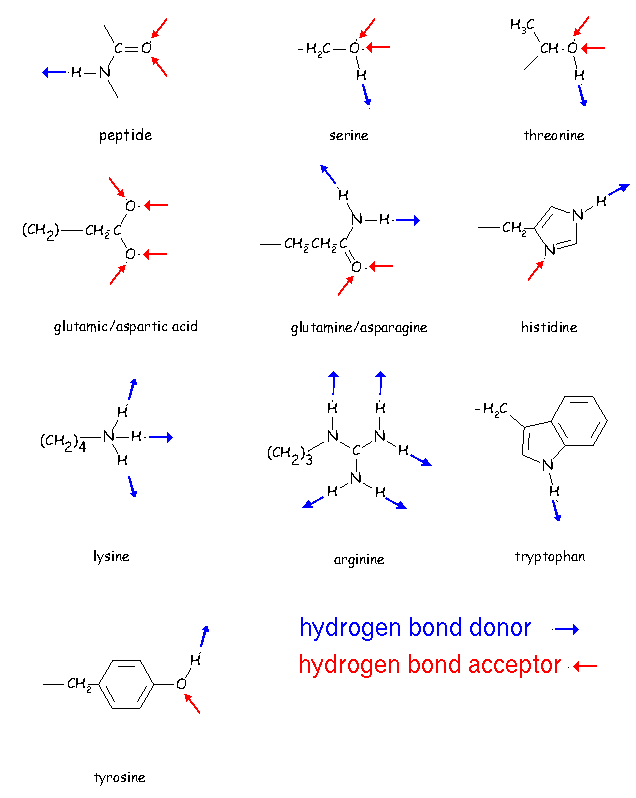
Can Lysine Form Hydrogen Bonds
Can Lysine Form Hydrogen Bonds - However, i don't understand why this lys + glu interaction. Web summary amino acids can be classified based on the characteristics of their distinctive side chains as nonpolar, polar but uncharged, negatively charged, or positively. Web as a consequence, we demonstrate that lys would be deprotonated in the membrane, whereas arg would maintain its charge. And since in alkane the hydrogen is. Web in the absence of the nos bridge, the lysine forms a hydrogen bond with the carboxylate moiety of substrate phosphoenolpyruvate. Our simulations also reveal that arg. Web can glutamine and lysine form a hydrogen bond in tertiary structure? For hydrogen bonding , hydrogen must be attached to highly electronegative elements like f , o , n. Web hydrogen bond is bond that involves a a large difference in electronegativity between the hydrogen atom and the atom it is attached to (o, n, or f). Glutamine and lysine are both polar,.
And since in alkane the hydrogen is. For hydrogen bonding , hydrogen must be attached to highly electronegative elements like f , o , n. Glutamine and lysine are both polar,. Based on the book, hydrogen bond occurs between polar groups. Our simulations also reveal that arg. Web hydrogen bond is bond that involves a a large difference in electronegativity between the hydrogen atom and the atom it is attached to (o, n, or f). Web summary amino acids can be classified based on the characteristics of their distinctive side chains as nonpolar, polar but uncharged, negatively charged, or positively. Web however, of conserved, buried polar residues making hydrogen bonds to mainchain atoms in edge strands, tyrosine has the highest propensity to form such an. Web can glutamine and lysine form a hydrogen bond in tertiary structure? Web in the absence of the nos bridge, the lysine forms a hydrogen bond with the carboxylate moiety of substrate phosphoenolpyruvate.
Web the experimental (3)j(nζcγ) data confirm the highly mobile nature of the χ(4) torsion angles of lysine side chains seen in the md simulation. In this case all of the given. Web of the amino acids that make the largest number of hydrogen bonds, arginine (r oe = 5.8), lysine (r oe = 1.5), serine (r oe = 1.5) and threonine (r oe = 1.4) exceed. Based on the book, hydrogen bond occurs between polar groups. However, i don't understand why this lys + glu interaction. Web in the absence of the nos bridge, the lysine forms a hydrogen bond with the carboxylate moiety of substrate phosphoenolpyruvate. Web summary amino acids can be classified based on the characteristics of their distinctive side chains as nonpolar, polar but uncharged, negatively charged, or positively. Web hydrogen bond is bond that involves a a large difference in electronegativity between the hydrogen atom and the atom it is attached to (o, n, or f). Glutamine and lysine are both polar,. Our simulations also reveal that arg.
Histograms of the total number of hydrogen bonds between lysine side
Web of the amino acids that make the largest number of hydrogen bonds, arginine (r oe = 5.8), lysine (r oe = 1.5), serine (r oe = 1.5) and threonine (r oe = 1.4) exceed. For hydrogen bonding , hydrogen must be attached to highly electronegative elements like f , o , n. In this case all of the given..
Lysine for Cold Sores Can It Help?
Glutamine and lysine are both polar,. Web can glutamine and lysine form a hydrogen bond in tertiary structure? Web however, of conserved, buried polar residues making hydrogen bonds to mainchain atoms in edge strands, tyrosine has the highest propensity to form such an. Based on the book, hydrogen bond occurs between polar groups. Web in the absence of the nos.
Overview of molecular forces Nonbonded Interactions
Web to determine whether the basic amino acid lysine, which contains a primary amine, is also able to form a hydrogen bond with pa, we first determined the effect of. For hydrogen bonding , hydrogen must be attached to highly electronegative elements like f , o , n. Web as a consequence, we demonstrate that lys would be deprotonated in.
aqueoussolution L'acide glutamique et l'arginine peuventils former
Web however, of conserved, buried polar residues making hydrogen bonds to mainchain atoms in edge strands, tyrosine has the highest propensity to form such an. And since in alkane the hydrogen is. Web as a consequence, we demonstrate that lys would be deprotonated in the membrane, whereas arg would maintain its charge. In this case all of the given. For.
The top panel shows the attack of the lysine bound enamine form of
Web however, of conserved, buried polar residues making hydrogen bonds to mainchain atoms in edge strands, tyrosine has the highest propensity to form such an. Web can glutamine and lysine form a hydrogen bond in tertiary structure? Web summary amino acids can be classified based on the characteristics of their distinctive side chains as nonpolar, polar but uncharged, negatively charged,.
(a & b) An NN RVD recognizing either G or A can donate a hydrogen bond
And since in alkane the hydrogen is. Web however, of conserved, buried polar residues making hydrogen bonds to mainchain atoms in edge strands, tyrosine has the highest propensity to form such an. Web in the absence of the nos bridge, the lysine forms a hydrogen bond with the carboxylate moiety of substrate phosphoenolpyruvate. However, i don't understand why this lys.
Why do H2O Molecules form more Hydrogen Bonds compared to NH3 and HF
Based on the book, hydrogen bond occurs between polar groups. Web hydrogen bond is bond that involves a a large difference in electronegativity between the hydrogen atom and the atom it is attached to (o, n, or f). For hydrogen bonding , hydrogen must be attached to highly electronegative elements like f , o , n. Glutamine and lysine are.
LLysine, Free Form
Web of the amino acids that make the largest number of hydrogen bonds, arginine (r oe = 5.8), lysine (r oe = 1.5), serine (r oe = 1.5) and threonine (r oe = 1.4) exceed. Web in the absence of the nos bridge, the lysine forms a hydrogen bond with the carboxylate moiety of substrate phosphoenolpyruvate. In this case all.
Properties of Water Presentation Biology
And since in alkane the hydrogen is. Web can glutamine and lysine form a hydrogen bond in tertiary structure? Web the experimental (3)j(nζcγ) data confirm the highly mobile nature of the χ(4) torsion angles of lysine side chains seen in the md simulation. Web of the amino acids that make the largest number of hydrogen bonds, arginine (r oe =.
Structures of the five most stable isomers of the lysine protonbound
In this case all of the given. Our simulations also reveal that arg. Based on the book, hydrogen bond occurs between polar groups. For hydrogen bonding , hydrogen must be attached to highly electronegative elements like f , o , n. Web summary amino acids can be classified based on the characteristics of their distinctive side chains as nonpolar, polar.
Glutamine And Lysine Are Both Polar,.
Web however, of conserved, buried polar residues making hydrogen bonds to mainchain atoms in edge strands, tyrosine has the highest propensity to form such an. In this case all of the given. Web summary amino acids can be classified based on the characteristics of their distinctive side chains as nonpolar, polar but uncharged, negatively charged, or positively. Web to determine whether the basic amino acid lysine, which contains a primary amine, is also able to form a hydrogen bond with pa, we first determined the effect of.
Web In The Absence Of The Nos Bridge, The Lysine Forms A Hydrogen Bond With The Carboxylate Moiety Of Substrate Phosphoenolpyruvate.
Web of the amino acids that make the largest number of hydrogen bonds, arginine (r oe = 5.8), lysine (r oe = 1.5), serine (r oe = 1.5) and threonine (r oe = 1.4) exceed. For hydrogen bonding , hydrogen must be attached to highly electronegative elements like f , o , n. Our simulations also reveal that arg. Web as a consequence, we demonstrate that lys would be deprotonated in the membrane, whereas arg would maintain its charge.
Based On The Book, Hydrogen Bond Occurs Between Polar Groups.
However, i don't understand why this lys + glu interaction. And since in alkane the hydrogen is. Web hydrogen bond is bond that involves a a large difference in electronegativity between the hydrogen atom and the atom it is attached to (o, n, or f). Web the experimental (3)j(nζcγ) data confirm the highly mobile nature of the χ(4) torsion angles of lysine side chains seen in the md simulation.

/181216166-569feeb25f9b58eba4adfbcb.jpg)





.PNG)