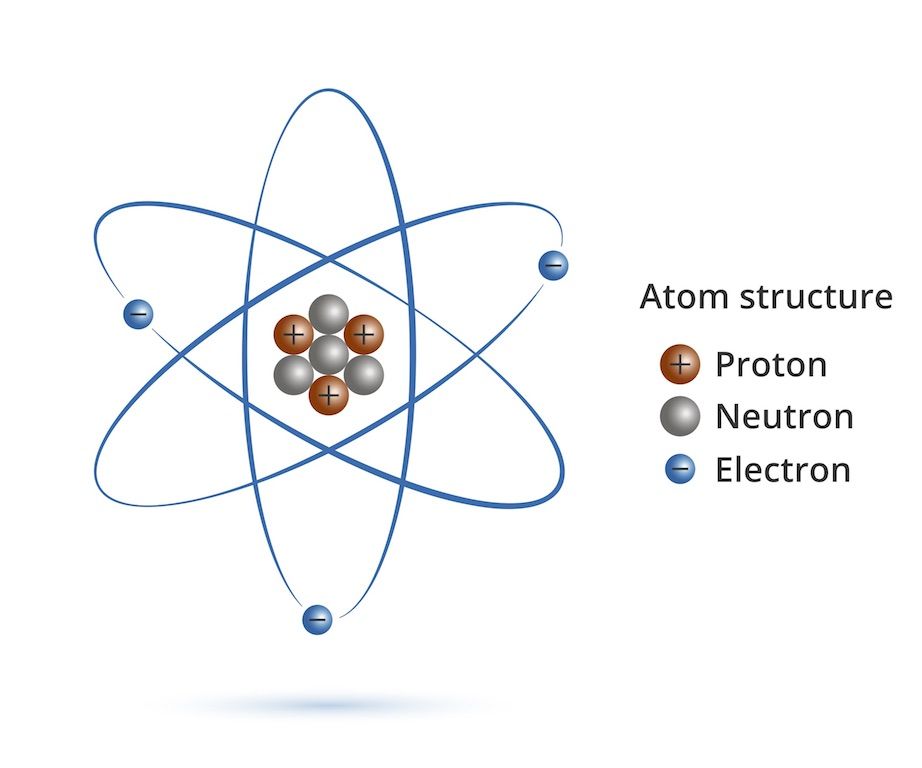
Atoms That Are The Same Form A
Atoms That Are The Same Form A - The three atoms bond together, forming a stable molecule. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Atoms with the same number of protons but different numbers of neutrons are called isotopes of the same chemical element. Basically, any material with a composition that includes more than one element symbol or that has a subscript. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Web elements are made up of particles called atoms. If a diatomic molecule consists of two atoms of the same element, such as hydrogen (h 2) or oxygen (o 2), then it is said to be homonuclear. Web because an atom usually has the same number of electrons as protons, the atomic number identifies the usual number of electrons as well. March 2, 2021 at 6:25 pm. Each atom of hydrogen has two electrons and the.
Employing standard techniques, the researchers used laser light to increase and. Ions similarly, atoms of the same element may have more or less electrons, and an atom with a net electric charge (i.e. Web home and his team used the shared vibrational motion of two ionized atoms—one calcium and one beryllium—to create their phonon laser. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). In a simplified model of a water molecule, two atoms of hydrogen share their valence electrons with an atom of oxygen. Web atoms always have an equal number of protons and electrons, and the number of protons and neutrons is usually the same as well. For instance, atoms might be connected by strong bonds and organized into molecules or crystals. Web beta created by beautybyvictoria18 terms in this set (15) the term used to identify anything that occupies space is called: Web atoms of the same element may have more or less neutrons, and the different atoms are referred to as isotopes. If a diatomic molecule consists of two atoms of the same element, such as hydrogen (h 2) or oxygen (o 2), then it is said to be homonuclear.
They are listed on the periodic table. Usually an atom has the same number of electrons as protons. As a consequence of sharing or exchanging electrons between the atoms, these bonds form. Web atoms of the same element may have more or less neutrons, and the different atoms are referred to as isotopes. Web the atoms in one element are all the same as each other, but they are different from the atoms of any other elements. The atoms could oscillate in their electric field trap like two coupled pendulums swinging back and forth. The total number of protons and neutrons determine the nuclide. Fundamental properties of atoms including atomic number and atomic mass. Dalton based his theory on the law of conservation of mass and the law of constant composition. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and.
Scientific Proof of God, A New and Modern Bible, and Coexisting
The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in. Web atoms of the same element may have more or less neutrons, and the different atoms are referred to as isotopes. Web atomic number, atomic mass, and isotopes. Web introduction only when two atoms of the same element form.
What Is A Half Life? How Elements With Short Half Lives Even Exist?
Not neutral) is referred to as an ion. Web all atoms are roughly the same size, whether they have 3 or 90 electrons. Web dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Web atoms of the same element may have more or less neutrons, and the different atoms are.
Are all atoms the same? AMAZING 8TH GRADE SCIENTISTS
In chemistry, an element is a pure substance consisting only of atoms that all have the same numbers of protons in their atomic nuclei. Web elements are made up of particles called atoms. Web home and his team used the shared vibrational motion of two ionized atoms—one calcium and one beryllium—to create their phonon laser. Web beta created by beautybyvictoria18.
Periodic Variations in Element Properties Chemistry I
Web atoms of the same element have the same number of protons, called the atomic number. Web the number of electrons in an atom is always the same as the number of protons, so atoms are electrically neutral. Web because an atom usually has the same number of electrons as protons, the atomic number identifies the usual number of electrons.
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology
Web because an atom usually has the same number of electrons as protons, the atomic number identifies the usual number of electrons as well. Web beta created by beautybyvictoria18 terms in this set (15) the term used to identify anything that occupies space is called: Web as atoms come together to form molecules, chemical bonds bind them together. An atom.
thinkbiggerdesigns Why Do Atoms Ions
Web atoms with the same number of protons belong to the same chemical element. There are 118 different elements. Web atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. Web beta created by beautybyvictoria18 terms in this set (15) the term used to identify anything that occupies space is called: Web home.
What Is an Atom? Live Science
Web beta created by beautybyvictoria18 terms in this set (15) the term used to identify anything that occupies space is called: Ions similarly, atoms of the same element may have more or less electrons, and an atom with a net electric charge (i.e. Web atoms of the same element may have more or less neutrons, and the different atoms are.
2.6 Molecules and Molecular Compounds Chemistry LibreTexts
Instead, they’re usually interacting with other atoms (or groups of atoms). In their most common form, many elements also contain the same number of neutrons as protons. Web it naturally attracts other atoms with unpaired electrons, such as hydrogen, which has only one electron. Some matter is either smaller or larger than an atom.examples of chemical species that are not.
Molecules and Compounds Definition, Differenences [in Table Form]
Atoms can lose or gain electrons. Web what are not atoms?. Web beta created by beautybyvictoria18 terms in this set (15) the term used to identify anything that occupies space is called: Not neutral) is referred to as an ion. A) a gas b) matter (correct) c) a solid d) organic matter with definite weight and volume but no definite.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
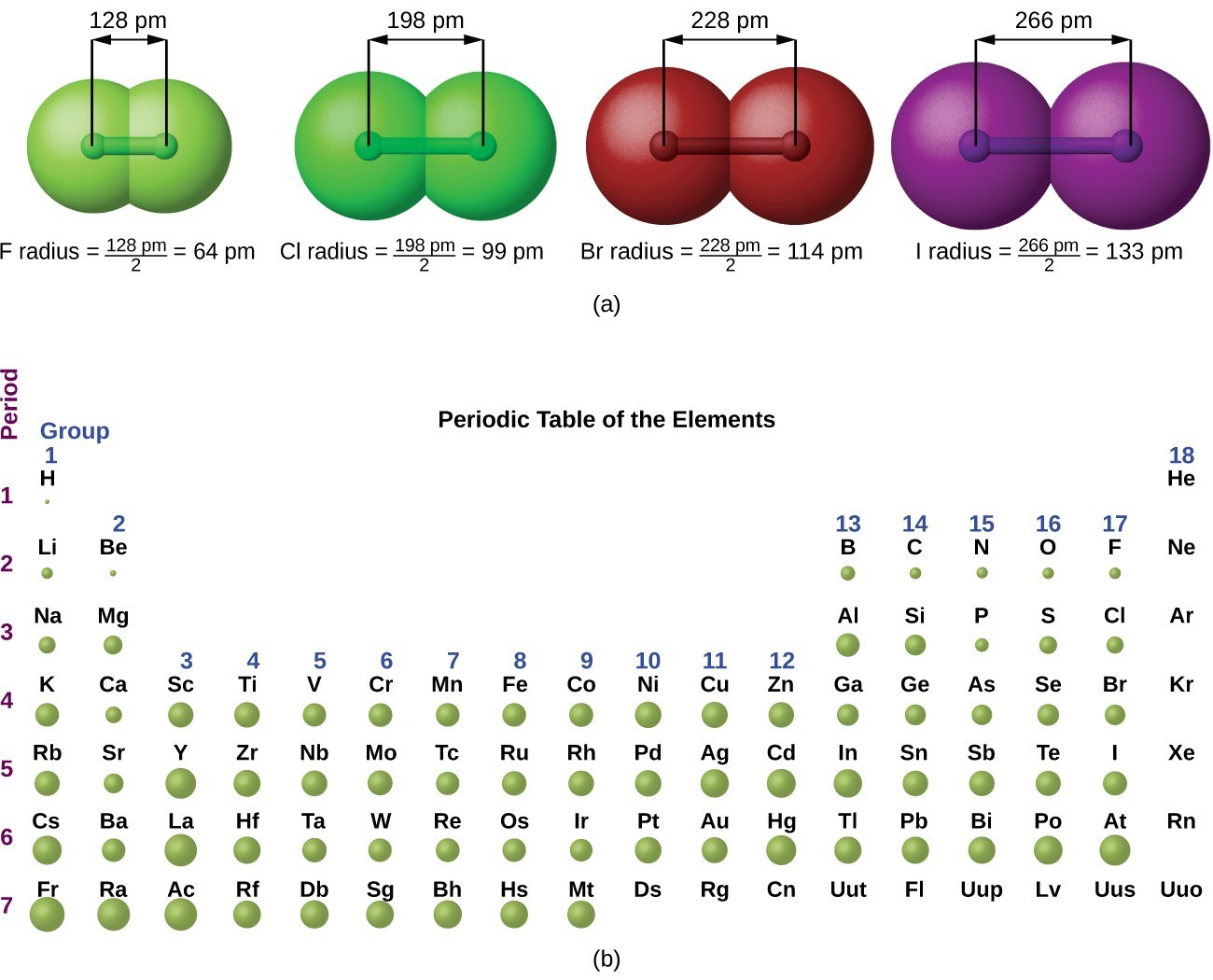
Atoms with the same number of protons, but different numbers of neutrons, are called isotopes. A convenient unit of length for measuring atomic sizes is the angstrom (å), defined as 10 −10 metre. Web both forms represent the same periodic table. Web because an atom usually has the same number of electrons as protons, the atomic number identifies the usual.
Molecules And Compounds Consist Of Atoms But Are Not Themselves Atoms.examples Of Molecules And.
Web home and his team used the shared vibrational motion of two ionized atoms—one calcium and one beryllium—to create their phonon laser. A) a gas b) matter (correct) c) a solid d) organic matter with definite weight and volume but no definite shape is. Adding a proton to an atom makes a new element, while adding a. The three atoms bond together, forming a stable molecule.
Atoms Can Lose Or Gain Electrons.
For instance, atoms might be connected by strong bonds and organized into molecules or crystals. A convenient unit of length for measuring atomic sizes is the angstrom (å), defined as 10 −10 metre. Usually an atom has the same number of electrons as protons. Web the number of electrons in an atom is always the same as the number of protons, so atoms are electrically neutral.
Web The Atoms In One Element Are All The Same As Each Other, But They Are Different From The Atoms Of Any Other Elements.
Atoms with the same number of protons, but different numbers of neutrons, are called isotopes. The difference between atom and element. Web atoms of the same element have the same number of protons, called the atomic number. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in.
Web Beta Created By Beautybyvictoria18 Terms In This Set (15) The Term Used To Identify Anything That Occupies Space Is Called:
March 2, 2021 at 6:25 pm. The total number of protons and neutrons determine the nuclide. Web what are not atoms?. A compound is a distinct group of atoms held together by chemical bonds.


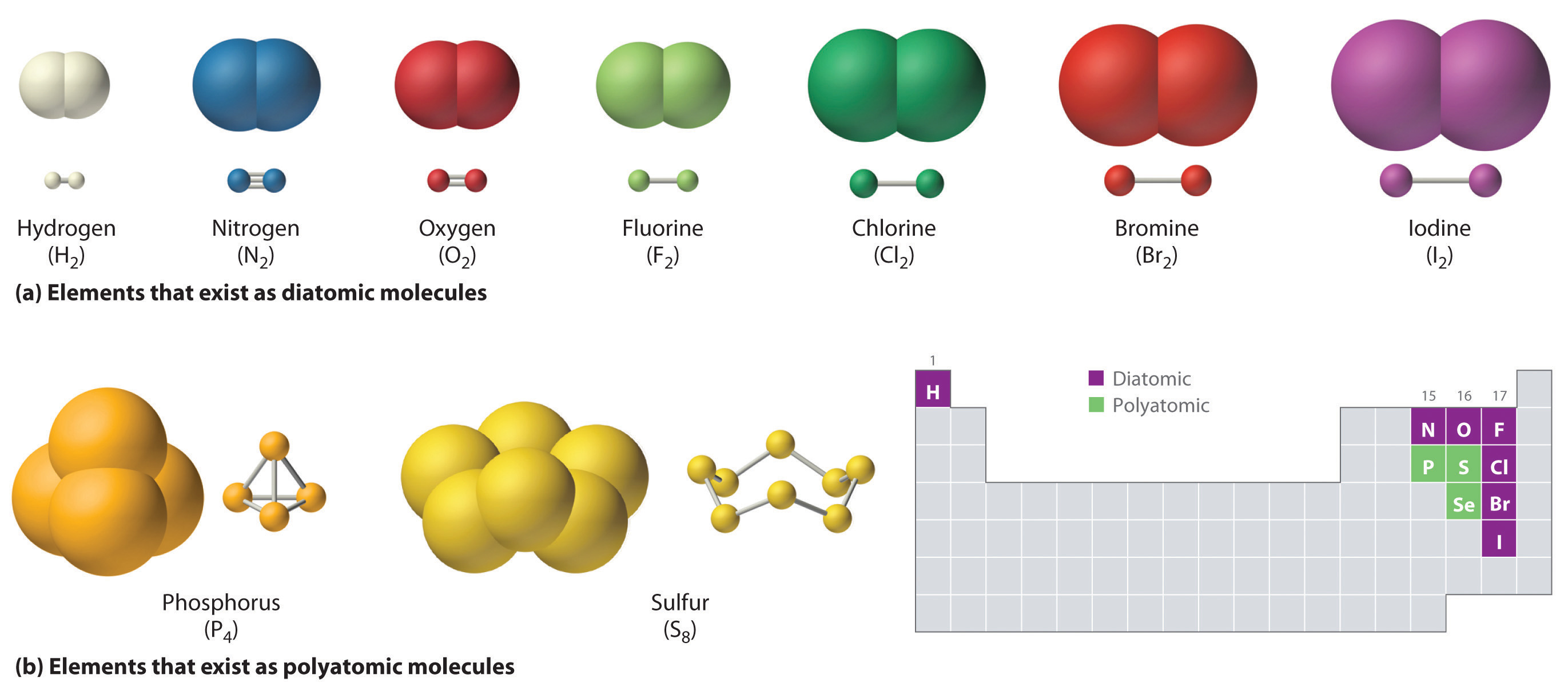
![Molecules and Compounds Definition, Differenences [in Table Form]](https://d1avenlh0i1xmr.cloudfront.net/large/756bdbc0-0026-418f-bc9f-de699cc72183/molecules-of-single-element-and-their-atomicity-teachoo-01.jpg)
