Arrhenius Equation Two Point Form
Arrhenius Equation Two Point Form - Ln k 2 k 1 = e a r ( 1 t 1 − 1. R.t take the natural log ln(k ) ln a e e a. Web the equation is commonly given in the form of an exponential function, k = a exp (− e / rt ), and it predicts that a small increase in reaction temperature will produce. This has the form y=mx. It uses two (any two points that fall on the line) and the slope of the line (therefore the name. Web an arrhenius plot plots the log or natural log of the measured parameter (p, d, or s) against the inverse absolute temperature (1/k). Contrast this with a second order reaction in (b) where during the first 2.5 s t 1/2, the concentration falls from 1.0m to 0.5m. R.t expand expression (multiplied inside log so add) ln(k ) ln(a ) ln e e a. Web use the 2 point form of the arrhenius equation to calculate k at 80.0 °c. Web the arrhenius equation k a e e a.
Web the equation is commonly given in the form of an exponential function, k = a exp (− e / rt ), and it predicts that a small increase in reaction temperature will produce. It uses two (any two points that fall on the line) and the slope of the line (therefore the name. Web two point arrhenius equation. Web how to calculate activation energy (ea) with arrhenius equation. Web the arrhenius equation for the activation energy of a chemical reaction, especially its two point form, is an intimidating looking beast. Web the arrhenius equation k a e e a. A) 1.5 x 10 5 kj/mol. R.t take the natural log ln(k ) ln a e e a. Ln k 2 k 1 = e a r ( 1 t 1 − 1. Alright, so now we have two different equations here for two.
Web so the natural log of k two is equal to negative ea over r times one over t two this time plus natural log of a. Web use the 2 point form of the arrhenius equation to calculate k at 80.0 °c. In physical chemistry, the arrhenius equation is a formula for the temperature dependence of reaction rates. This has the form y=mx. Contrast this with a second order reaction in (b) where during the first 2.5 s t 1/2, the concentration falls from 1.0m to 0.5m. It uses two (any two points that fall on the line) and the slope of the line (therefore the name. This equation has a vast and important application in determinin… Now here we're going to say that. The equation was proposed by svante arrhenius in 1889, based on the work of dutch chemist jacobus henricus van 't hoff who had noted in 1884 that the van 't hoff equation for the temperature dependence of equilibrium constants suggests such a formula for the rates of both forward and reverse reactions. Ln k 2 k 1 = e a r ( 1 t 1 − 1.
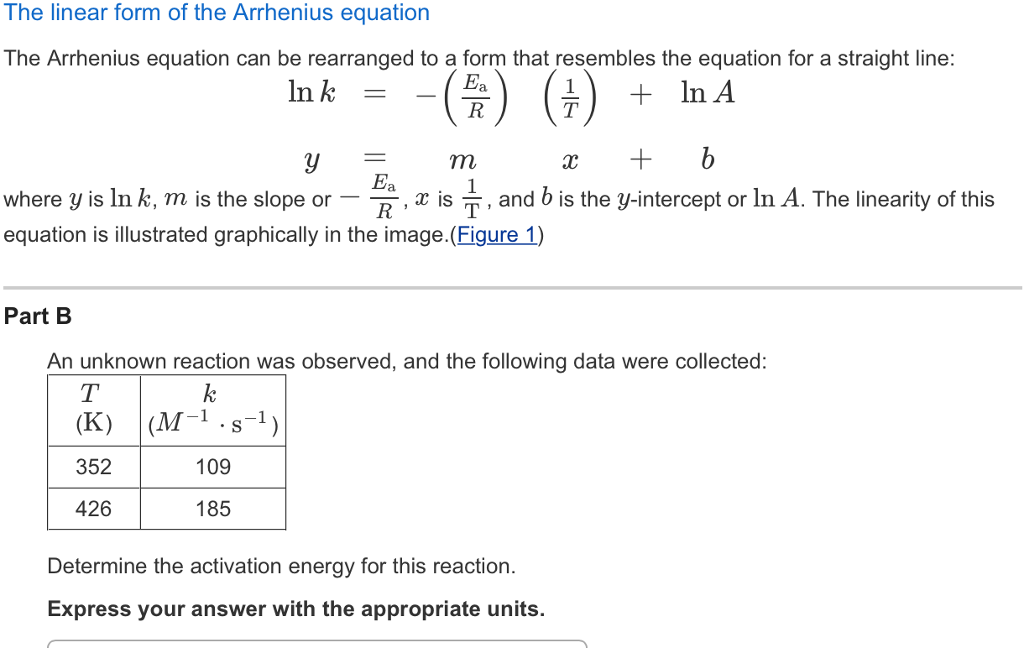
Solved The linear form of the Arrhenius equation The
This equation has a vast and important application in determinin… Web the arrhenius equation for the activation energy of a chemical reaction, especially its two point form, is an intimidating looking beast. Web two point arrhenius equation. R.t expand expression (multiplied inside log so add) ln(k ) ln(a ) ln e e a. What is the activation energy?
Chapter13 chemical
Contrast this with a second order reaction in (b) where during the first 2.5 s t 1/2, the concentration falls from 1.0m to 0.5m. Web two point arrhenius equation. However the second t 1/2 takes 5 s. Web use the 2 point form of the arrhenius equation to calculate k at 80.0 °c. Web the arrhenius equation for the activation.
Two point arrhenius equation, Easy derivation, 3 application
Web now the two point form of the iranians equation shows how changing the temperature can impact the rate constant which uses the variable que. Contrast this with a second order reaction in (b) where during the first 2.5 s t 1/2, the concentration falls from 1.0m to 0.5m. However the second t 1/2 takes 5 s. R.t expand expression.
arrhenius equation Yahoo Image Search Results Energy Activities
In physical chemistry, the arrhenius equation is a formula for the temperature dependence of reaction rates. However the second t 1/2 takes 5 s. Web the arrhenius equation k a e e a. The equation was proposed by svante arrhenius in 1889, based on the work of dutch chemist jacobus henricus van 't hoff who had noted in 1884 that.
16.2 The Arrhenius equation (HL) YouTube
Alright, so now we have two different equations here for two. It uses two (any two points that fall on the line) and the slope of the line (therefore the name. Web how to calculate activation energy (ea) with arrhenius equation. In physical chemistry, the arrhenius equation is a formula for the temperature dependence of reaction rates. 721 views 2.
13.4 The Arrhenius Equation YouTube
Alright, so now we have two different equations here for two. A) 1.5 x 10 5 kj/mol. Web an arrhenius plot plots the log or natural log of the measured parameter (p, d, or s) against the inverse absolute temperature (1/k). Contrast this with a second order reaction in (b) where during the first 2.5 s t 1/2, the concentration.
TwoPoint Form for Arrhenius Equation YouTube
This has the form y=mx. Web use the 2 point form of the arrhenius equation to calculate k at 80.0 °c. However the second t 1/2 takes 5 s. It uses two (any two points that fall on the line) and the slope of the line (therefore the name. R.t take the natural log ln(k ) ln a e e.
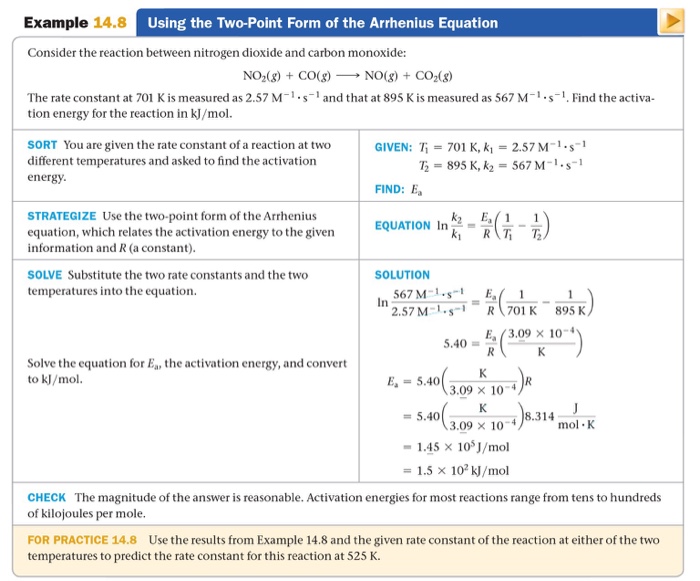
I Need Help With For Practice 14.8. I'm Not Sure H...
At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can. Now here we're going to say that. Web so the natural log of k two is equal to negative ea over r times one over t two this time plus natural log of a..
Chapter13 chemical
Web the equation is commonly given in the form of an exponential function, k = a exp (− e / rt ), and it predicts that a small increase in reaction temperature will produce. Now here we're going to say that. Ln k 2 k 1 = e a r ( 1 t 1 − 1. A) 1.5 x 10.
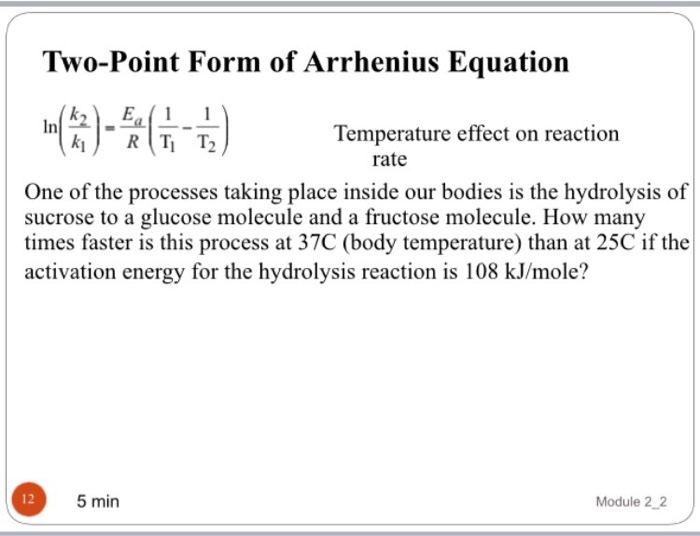
Solved TwoPoint Form of Arrhenius Equation In N2.
Web the equation is commonly given in the form of an exponential function, k = a exp (− e / rt ), and it predicts that a small increase in reaction temperature will produce. When plotted in this manner, the value of. What is the activation energy? The equation was proposed by svante arrhenius in 1889, based on the work.
This Has The Form Y=Mx.
What is the activation energy? In physical chemistry, the arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by svante arrhenius in 1889, based on the work of dutch chemist jacobus henricus van 't hoff who had noted in 1884 that the van 't hoff equation for the temperature dependence of equilibrium constants suggests such a formula for the rates of both forward and reverse reactions. Web how to calculate activation energy (ea) with arrhenius equation.
Web Use The 2 Point Form Of The Arrhenius Equation To Calculate K At 80.0 °C.
It uses two (any two points that fall on the line) and the slope of the line (therefore the name. Now here we're going to say that. R.t expand expression (multiplied inside log so add) ln(k ) ln(a ) ln e e a. Web two point arrhenius equation.
However The Second T 1/2 Takes 5 S.
Alright, so now we have two different equations here for two. Web the arrhenius equation for the activation energy of a chemical reaction, especially its two point form, is an intimidating looking beast. Web the equation is commonly given in the form of an exponential function, k = a exp (− e / rt ), and it predicts that a small increase in reaction temperature will produce. 721 views 2 years ago general.
R.t Take The Natural Log Ln(K ) Ln A E E A.
This equation has a vast and important application in determinin… Web now the two point form of the iranians equation shows how changing the temperature can impact the rate constant which uses the variable que. Web so the natural log of k two is equal to negative ea over r times one over t two this time plus natural log of a. A) 1.5 x 10 5 kj/mol.